ATCC Primary Cell Solutions
Guide to culturing human primary cells
Primary cells and cell types
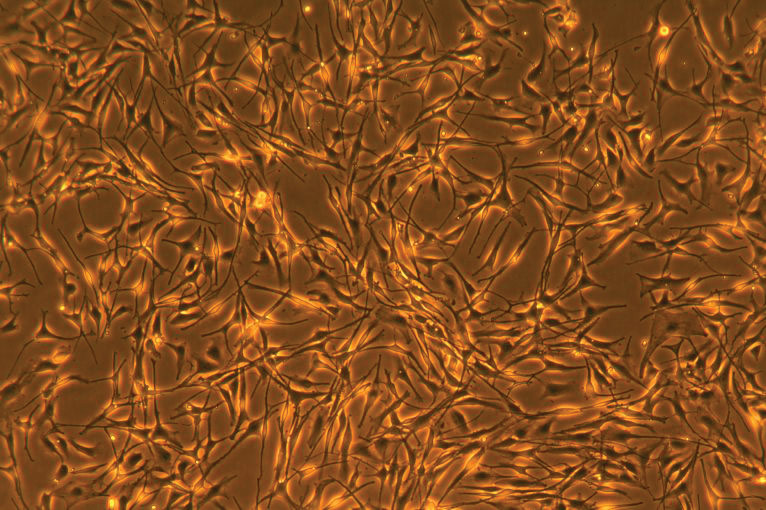
Primary cell cultures more closely mimic the physiological state of cells in vivo and generate more relevant data representing living systems. Primary cultures consist of cells that have been freshly derived from a living organism and are maintained for growth in vitro. Primary cells can be categorized according to the genus from which they are isolated, as well as by species or tissue type. Each mammalian tissue type is derived from the embryonic germ layer consisting of ectoderm, endoderm and mesoderm, which differentiate into the many cell types that organize into tertiary structures such as skin, muscle, internal organs, bone and cartilage, the nervous system, blood and blood vessels.1 The cell types most frequently found in primary cell culture are epithelial cells, fibroblasts, keratinocytes, melanocytes, endothelial cells, muscle cells, hematopoietic and mesenchymal stem cells.
Basic properties of primary cells
Once adapted to in vitro culture conditions, primary cells undergo a limited, predetermined number of cell divisions before entering senescence. The number of times a primary cell culture can be passaged is minimal due to the Hayflick Limit, nutrient requirements and culture conditions, and the expertise by which they are manipulated and subcultured. In contrast, cell lines that have been immortalized by viral, hTERT, or tumorigenic transformation typically undergo unlimited cell division and have an infinite lifespan. And, unlike tumor cell lines cultured in medium containing 10% to 20% serum, primary cell cultures are fastidious, requiring optimized growth conditions, including the addition of tissue specific cytokines and growth factors for propagation in serum-free or low-serum growth media.
Benefits of primary cells
Primary cell cultures are commonly used as in vitro tools for preclinical and investigative biological research, such as studies of inter- and intracellular communication, developmental biology, and elucidation of disease mechanisms, such as cancer, Parkinson’s disease, and diabetes. Historically, investigators have employed immortalized cell lines in research related to tissue function; however, the use of cell lines containing gross mutations and chromosomal abnormalities provides poor indicators of normal cell phenotype and progression of early-stage disease. The use of primary cells, maintained for only short periods of time in vitro, now serves as the best representative of the main functional component of the tissue (in vivo) from which they are derived.
Isolation of primary cells
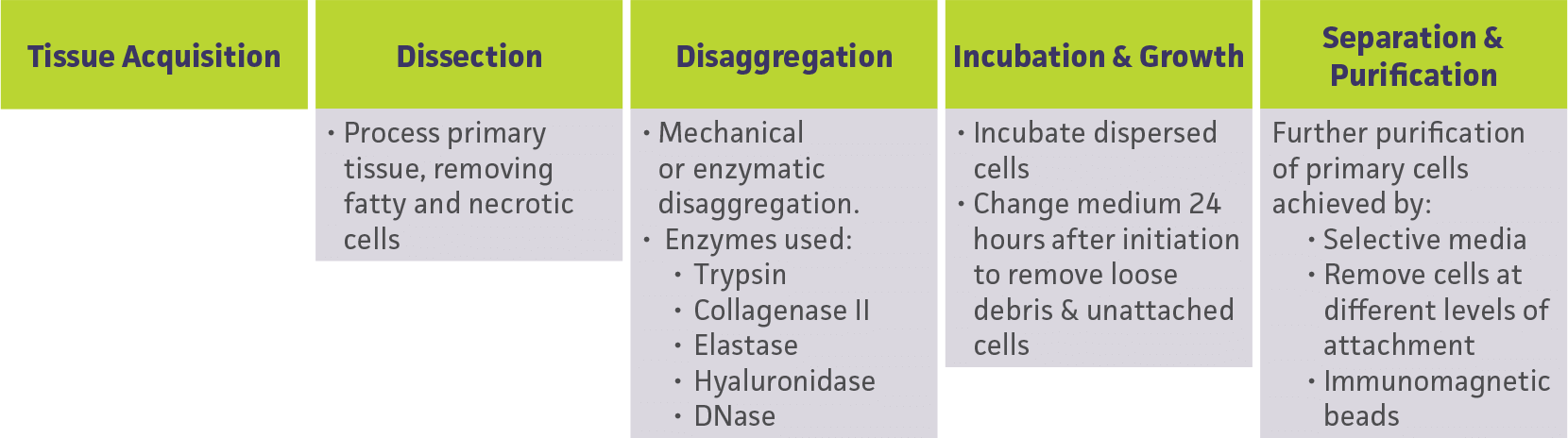
The isolation and purification of peripheral blood cells can be easily achieved by differential centrifugation or by positive sorting using magnetic beads. On the other hand, the isolation of a pure population of cells from primary tissue is often difficult to perform, and requires knowledge of how the cell strata should be teased apart into a suspension containing only one predominant cell type. Diagram 1 is an illustration of some of the basic steps used to establish a primary cell culture14.
Primary cell culture
Growth requirements
Primary cells, except for those derived from peripheral blood, are anchorage-dependent, adherent cells, meaning they require a surface in order to grow properly in vitro. In most cases, primary cells are cultured in a flat, uncoated, plastic vessel, but sometimes a microcarrier, which can greatly increase the surface area, can be used. A complete cell culture media, composed of a basal medium supplemented with appropriate growth factors and cytokines, is required. During establishment of primary cultures, it may be useful to include an antibiotic in the growth medium to inhibit contamination introduced from the host tissue. These may include a mixture of gentamicin, penicillin, streptomycin, and amphotericin B. However, long-term use of antibiotics is not advised, since some reagents, such as amphotericin B, may be toxic to cells over time.
Diagram 1: Basic steps used to isolate cells from primary tissue
Maintenance
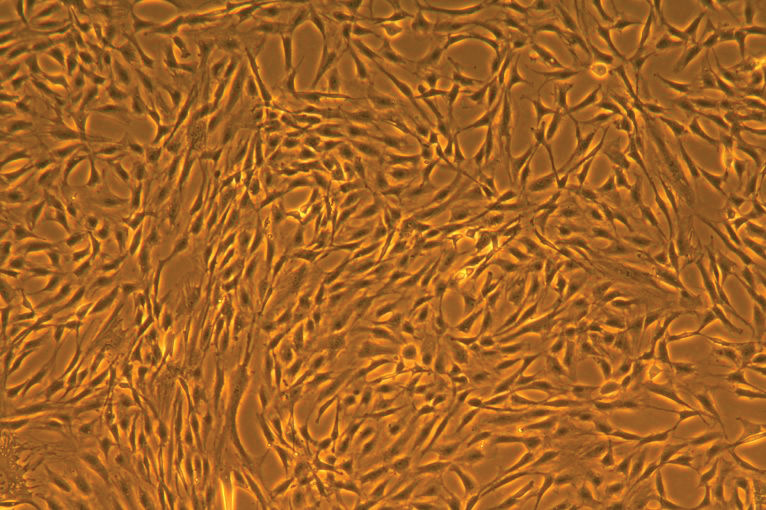
The maintenance phase begins when cells have attached to the surface of the culture dish. Attachment usually occurs about 24 hours after initiation of the culture. When initiating a culture of cryopreserved primary cells, it is important to remove the spent media once the cells have attached because DMSO is harmful to primary cells and may cause a drop in post-thaw viability. When cells have reached a desired percent of cellular confluence and are actively proliferating, it is time to subculture. It is best to subculture primary cell cultures before reaching 100% confluence, since post-confluent cells may undergo differentiation and exhibit slower proliferation after passaging.
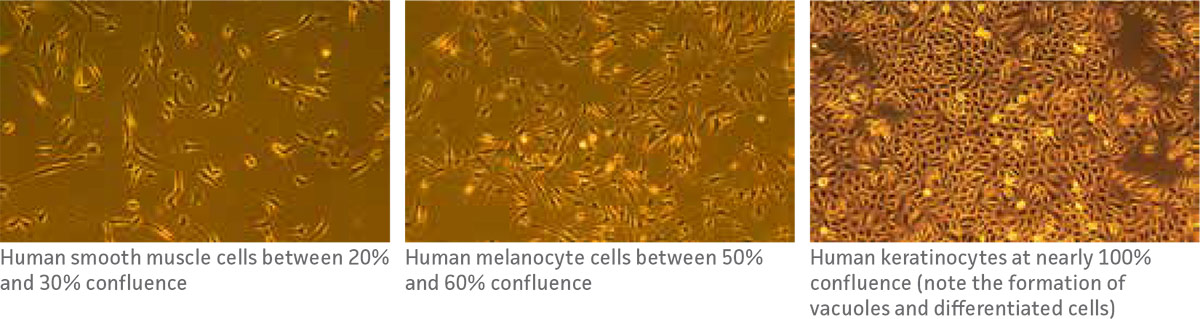
Cellular confluence
Cellular confluence refers to the percentage of the culture vessel inhabited by attached cells. For example, 100% cellular confluence means the surface area is completely covered by cells, while 50% confluence means roughly half of the surface is covered. It is an important parameter to track and assess in primary cell culture because different cell types require different confluence end points, at which point they need to be subcultured.
Levels of cellular confluence
Subculture
Anchorage-dependent cells grow in monolayers and need to be subcultured at regular intervals to maintain exponential growth. Subculturing procedures, including recommended split-ratios and medium replenishment (feeding) schedules for each ATCC primary cell culture, are provided on the product information sheet provided with each cell (available at www.atcc.org). Subcultivation of monolayers involves the breakage of both inter- and intracellular cell-to-surface bonds. Most adherent primary cells require the digestion of their protein attachment bonds with a low concentration of a proteolytic enzyme such as trypsin/EDTA. After the cells have been dissociated and dispersed into a single-cell suspension, they are counted and diluted to the appropriate concentration and transferred to fresh culture vessels where they will reattach and divide.
Cell counting
Hemocytometers are commonly used to estimate cell number and determine cell viability with the aid of an exclusion dye such as Trypan Blue or Erythrosin B. A hemocytometer is a fairly thick glass slide with two counting chambers, one on each side. Each counting chamber has a mirrored surface with a 3 × 3 mm2 grid consisting of 9 counting squares. The chambers have raised sides that will hold a coverslip exactly 0.1 mm2 above the chamber floor. Each of the 9 counting squares holds a volume of 0.0001 mL. Alternatively, counting cells can also be achieved by using an automated cell counter, such as Vi-CELL.
For a detailed description of cell counting using a hemocytometer, refer to the ATCC Animal Cell Culture Guide.
Cryopreservation and recovery
Special attention is needed to cryopreserve and thaw primary cells in order to minimize cell damage and death during each process. Cryopreservation of human cells is best achieved with the use of a cryoprotectant, such as DMSO or glycerol. Most primary cell cultures can be cryopreserved in a mixture of 80% complete growth medium supplemented with 10% FBS and 10% DMSO. The freezing process should be slow, at a rate of -1°C per minute, to minimize the formation of ice crystals within the cells. Once frozen, cultures are stored in the vapor phase of liquid nitrogen, or below -130°C.
Additional information about freezing cells can be found in the ATCC Technical Document: Cryogenic Storage of Animal Cells.
Thawing cryopreserved cells is a rapid process and is accomplished by immersing frozen cells in a 37°C water bath for about 1 to 2 minutes. Care should be taken not to centrifuge primary cells upon thaw, since they are extremely sensitive to damage during recovery from cryopreservation. It is best to plate cells directly upon thaw, and allow cultures to attach for the first 24 hours before changing the medium to remove residual DMSO.
Challenges of primary cell isolation and culture
There are several challenges associated with the use of primary cells. One of the greatest hurdles primary cell culturists face is limited cell accessibility due to issues with donor tissue supply, difficulty with cell isolation/purification, quality assurance and consistency, and contamination risks. Data comparability is also a serious issue with primary cell use, and arises out of variability among reagents used and the procedures implemented by individual scientists and laboratories to isolate and culture primary cells. A comparison between primary cells and continuous cell lines is described in Table 1.
Table 1: Comparison between primary cells and continuous cell lines
| Properties | Primary cells | Continuous cell lines |
|---|---|---|
| Life span and cell proliferation | Finite; limited to a small number of cell doublings | Infinite when handled properly |
| Consistency | Variability exists between donors and preparations | Minimal variability |
| Genetic integrity | Retains in vivo tissue genetic makeup through cell doublings | Subject to genetic drift as cells divide |
| Biological relevance | More closely mimics the physiology of cells in vivo | Relevance can drift over time as cells divide |
| Ease of use (freeze-thaw and use) | Requires optimized culture conditions and careful handling | Well established conditions and robust protocols exist |
| Time and expense to use | More time and less abundance of cells | Less time and more abundance of cells |
ATCC Primary Cell Solutions
ATCC has provided a solution to help investigators overcome the high cost and inconsistency found in routine primary cell culture with the development of ATCC Primary Cell Solutions, a standardized cell culture system that includes high-quality cells, media, supplements, reagents, and protocols. ATCC Primary Cell Solutions focuses on providing researchers with superior quality from a trusted source—each lot of ATCC Primary Cell Solutions primary cells is:
Cryopreserved at early passage
- ATCC Primary Cell Solutions primary cells are frozen at passages 1 through 3.
- Early passage material ensures higher viability and optimal plating efficiency.
Performance tested
- ATCC Primary Cell Solutions cells, media, kit supplements, and reagents are tested for optimal reliability.
- Growth and morphology are assessed to ensure all components work synergistically.
Quality controlled to ensure safety, purity, and functionality
- Sterility testing – All cells are tested for bacteria, yeast, fungi, and Mycoplasma.
- Viral testing – HIV-1, HIV-2, HBV, and HCV tissue screening is performed at isolation.
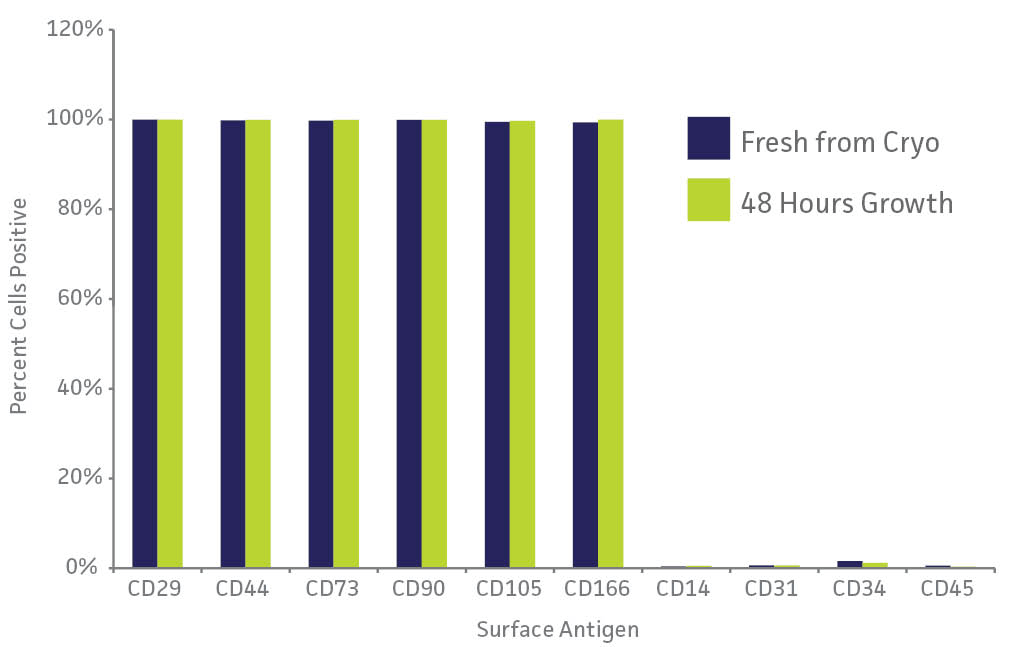
- Viability and growth – Viability and growth of each lot of cells is checked before freezing and after-thawing.
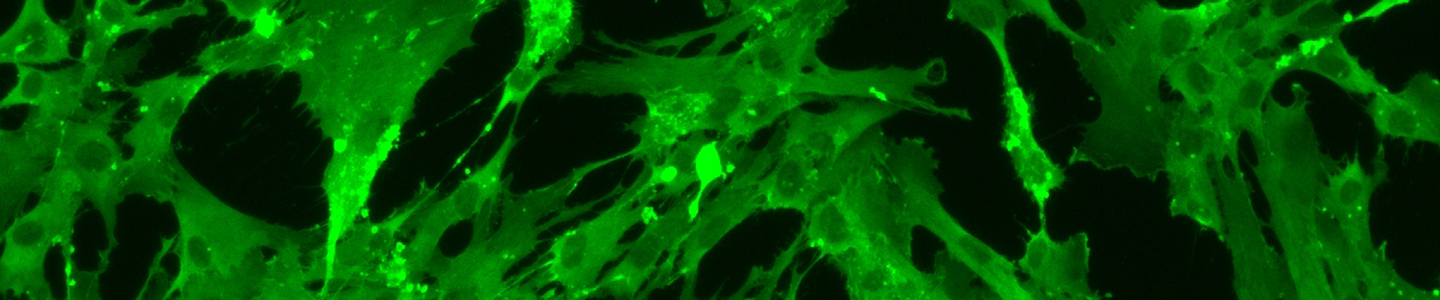
- Staining – Staining for cell-specific marker expression is determined and performed for some cell types.
ATCC Primary Cell Solutions basal media and cell-specific growth kits are designed to support recovery and proliferation of ATCC Primary Cell Solutions primary cells in vitro. Used as a system, ATCC Primary Cell Solutions primary cells, basal media, and cell-specific growth kits provide all the components needed to be successful in primary cell research. (Detailed formulations for each growth kit can be found at www.atcc.org.)