Accelerate your vaccine and gene therapeutic development projects
The continual spread of deadly viruses necessitates the development of novel prevention and treatment options; however, the development of a new antiviral vaccine can be challenged by low-yielding manufacturing processes. Similarly, the development of gene therapies is constrained during the large-scale production of adeno-associated virus (AAV) delivery platforms for gene transfer.
To speed the pace of these various areas of bioproduction, ATCC addressed the need for efficient viral replication by optimizing three cell lines commonly used in virus manufacturing. Here, we used CRISPR/Cas9 gene-editing technology to develop STAT1 and BAX knockout cell lines capable of producing high-titer viral stocks. These newly created cell lines can produce model clinical viruses and adeno-associated viruses at titers much higher than the parental cell lines, providing an efficient method for biopharmaceutical companies to increase production while reducing associated costs. Discover how these advanced biological models can be used in your vaccine and gene therapeutic development projects.
Explore our related resources
Vaccine Development
ATCC provides the virus-producing cell lines, microbial strains, and pneumococcal polysaccharides needed for vaccine research and development.
Cell Bank Production
We have the facilities and the expertise to expand cell lines to create master and working cell banks for your assay needs.
Reduce the costs associated with generating viral vaccines and gene therapies
ATCC’s cell lines for enhanced viral production have broad applicability for bioproduction in basic research and the pharmaceutical industry. Because of their ability to yield higher virus titers than their wild-type counterparts, these enhanced virus-producing cell lines have the potential to significantly reduce the costs associated with generating viral vaccines and gene therapies.
- 2 cell lines optimized for vaccine manufacturing
- 1 cell line optimized for gene therapy delivery
- Superior clinical virus or AAV yields compared to parental lines
- 10 to 30-fold higher virus yields possible
- Broad applicability for basic research or industrial scale manufacturing
- Gene-edited for precise and stable STAT1 and STAT1/BAX knockouts
- Similar growth profiles to the parental cell lines
- Scalable for large- to small-scale production
- cGMP-compliant custom services available
- ATCC quality and performance
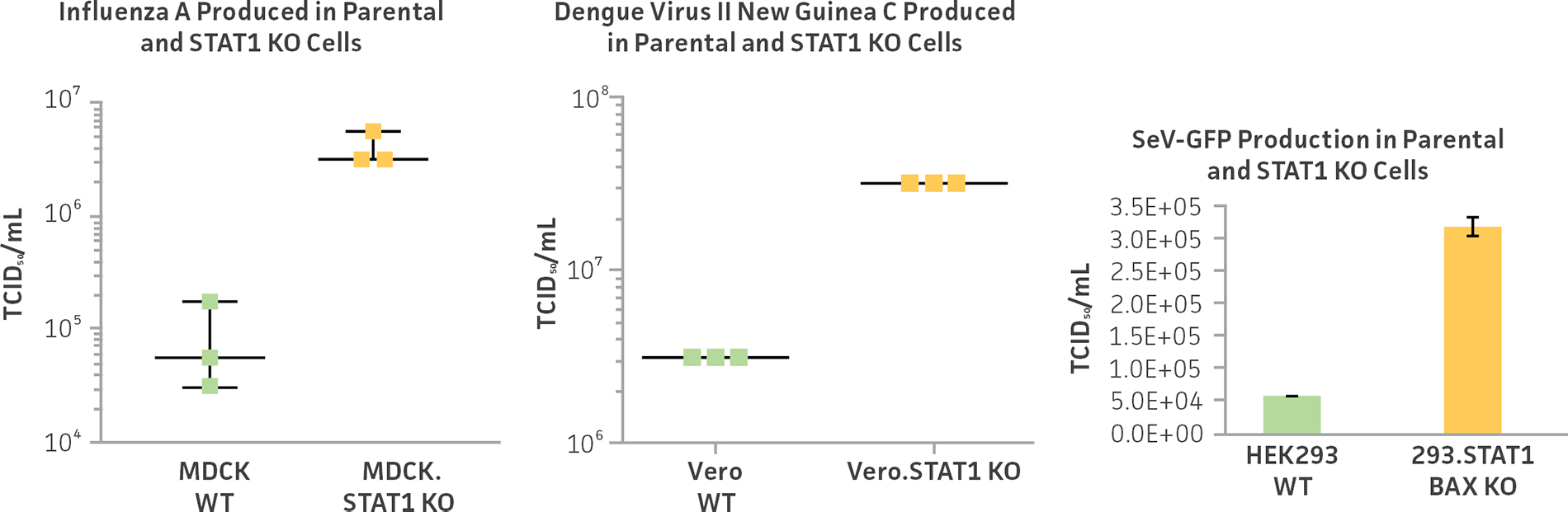
Figure 1: ATCC CRISPR-edited virus-producing cell lines show superior virus yields compared to parental cell lines. Staining of TCID50 of viral supernatants from MDCK.STAT1 KO, Vero.STAT1 KO, and 293.STAT1 BAX KO show a 10-fold increase in Influenza A virus production, Dengue virus type II production, and Sendai virus production over their respective parental cell lines
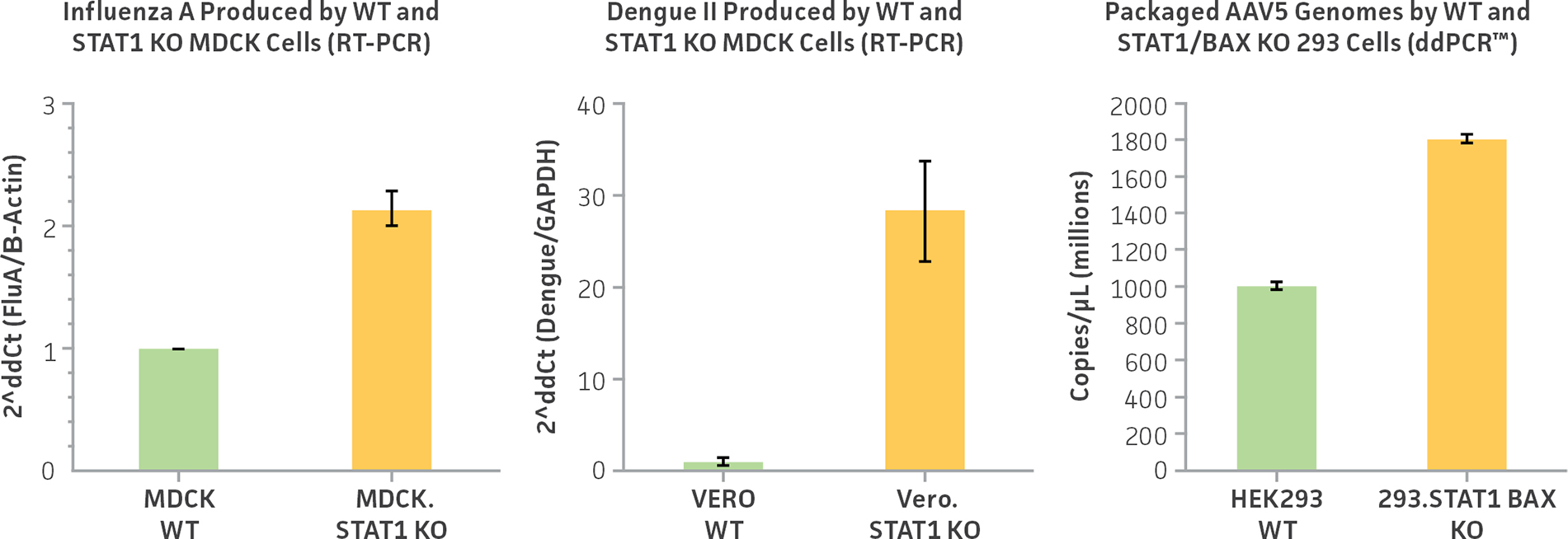
Figure 2: ATCC CRISPR-edited virus-producing cell lines show increase virus genome copy number compared to parental cell lines. Staining of TCID50 of viral supernatants from MDCK.STAT1KO, Vero.STAT1KO, and 293.STAT1 BAX KO show a 2-fold increase in Influenza A virus production, a 30-fold increase in Dengue virus type II production, and a 1.8-fold increase Sendai virus production over their respective parental cell lines.