Get tips and techniques for culturing hTERT-immortalized cells and ensure that your research is reproducible.
Table of Contents
Telomeres and Telomerase
ATCC hTERT-immortalized Cell Solutions
ATCC hTERT-immortalized Barrett’s Esophageal Epithelial Cells
ATCC hTERT-immortalized Bronchial Epithelial Cells
ATCC hTERT-immortalized Chondrocyte Fibroblast Cells
ATCC hTERT-immortalized Dermal Microvascular Endothelial Cells
ATCC hTERT-immortalized Endometrial Fibroblasts
ATCC hTERT-immortalized Human Foreskin Fibroblasts
ATCC hTERT-immortalized Mammary Epithelial Cells
ATCC hTERT-immortalized Pancreas Duct Epithelial Cells
ATCC hTERT-immortalized Renal Epithelial Cells
ATCC hTERT-immortalized Retinal Pigmented Epithelial Cells
Appendix
Tissue Types
References
Download a PDF of our hTERT-immortalized cell culture guide
Download Now
Telomeres and Telomerase
Telomeres are repetitive DNA sequences that stabilize the terminal ends of the chromosomes. During each cell division, 50-200 base pairs of DNA are lost from the telomere ends of the chromosomes, and chromosomal shortening eventually leads to replicative senescence.1 Telomerase is an enzyme, comprising an RNA component (ie, hTERC or hTR) and a catalytic component (ie, hTERT), that is able to restore the DNA base pairs lost from the telomeres during cell division. In cells with active telomerase, chromosomal length is maintained and the cells continue to divide without becoming senescent.1
The RNA component of telomerase is expressed ubiquitously2, while the expression of the catalytic hTERT component is mainly limited to the early stages of embryonic development, during which time it is expressed by stem cells. In the adult, its expression is restricted to some rare cells of the blood (ie, white blood cells), germ cells, and some cells of the skin and digestive track. Since most normal somatic cells do not have active telomerase, these cells are susceptible to replicative senescence in vivo and are difficult to maintain in vitro.1
hTERT immortalization of primary cells
Transfection of hTERT into human primary cells leads to elongation and maintenance of the telomere ends of the chromosomes. In many instances, forced expression of hTERT alone enables the cells to repress replicative senescence and overcome the growth crisis, effectively leading to their immortalization.2,3,4 In some cases, more than one immortalization agent may be required to successfully immortalize a particular cell type. For example primary cell lines may be immortalized using a combination of hTERT with one or more of the following: genes encoding viral [simian virus 40 (SV40) large T antigen5,6 and human papilloma virus-16 (HPV-16) E6/E77,8] or non-viral (Cdk-49 and Bmi-110) oncoproteins.
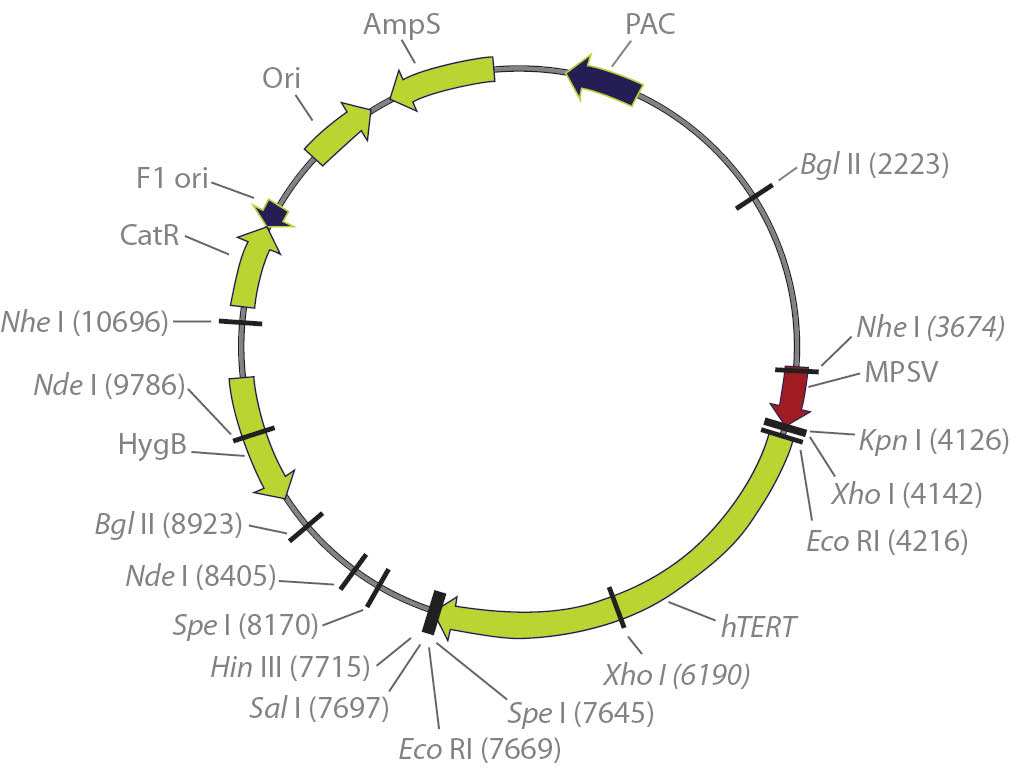
Figure 1. Eukaryotic expression plasmid containing the hTERT reverse transcriptase cDNA.16
hTERT-immortalized cells are mostly diploid, but may become pseudo-diploid especially at high passage number. In many cases, when cells become pseudo-diploid they still retain most primary cell functions (Table 1). The following is a list of primary human cells that have been established by the forced expression of hTERT alone.
- Endothelial cells12
- Esophageal squamous cells2
- Fibroblasts11
- Keratinocytes14
- Mammary epithelial cells13
- Nestin-positive cells of the pancreas16
- Osteoblasts15
- Retinal pigmented epithelial cells3
Properties of hTERT-immortalized cells
Early-passage hTERT-immortalized cells faithfully represent the physiological properties of normal cells in vivo. Additionally, analysis of numerous hTERT-immortalized cell lines has confirmed that these cells retain the expression of phenotypic markers and have a stable karyotype.13,14 This is in contrast to many traditional lines of immortalized cells, which are known to develop an unstable karyotype, especially at high passage numbers and when oncogenes are used. Below is a summary of the commonly observed properties of cells immortalized by the ectopic expression of hTERT.
- Nonmalignant17,19
- Normal cell cycle controls, functional p53 and pRB checkpoints17,18
- Contact inhibited19
- Anchorage dependent19
- Retain normal growth responses to serum and mitogens3
- Require growth factors for proliferation3
- Possess a normal karyotype4,18,*
- Do not show changes associated with transformation such as tumorigenicity or growth in soft agar20
Benefits of hTERT-immortalized cells
Primary cells closely represent the physiological state of a particular cell type in vivo, but they are susceptible to replicative senescence, so their value in the laboratory setting is limited. This is especially true when large quantities of cells are required for biochemical analysis, genetic manipulations or for genetic screens. It is also a factor for the study of some types of rare hereditary human diseases, since the volume of the biological samples collected (biopsies or blood) is usually small and contains a limited number of cells. Continuous cell lines, on the other hand, are not encumbered by replicative senescence, but, they often contain numerous genetic mutations, exhibit an unstable karyotype and have protein expression patterns that are not comparable with the cell type they are intended to represent.
hTERT cells combine the physiological attributes of primary cell lines and the long culture life of continuous cell lines, while avoiding the replicative senescence of the former and the unstable karyotype of the latter. Additionally, in many studies hTERT-immortalized cells have been induced to become differentiated cell types, exhibiting tissue-specific features, differentiation-specific proteins, and forming structures that resemble those formed in vivo.17
Table 1. Comparison between hTERT-immortalized cells, primary cells, oncogene/viral-immortalized cells, and continuous cell lines
| Primary cells | hTERT-immortalized | Onco, viral-immortalized | Continuous | |
|---|---|---|---|---|
| Mimic in vivo Tissue Phenotype | ++++ | +++ | ++ | + |
| Karyotypic Stability | Diploid | Diploid/ Pseudo-diploid | Pseudo-diploid/ Aneuploid | Aneuploid |
| Proliferative Capacity | + | +++ | +++ | +++ |
| Supply | + | +++ | +++ | +++ |
| Inter-Experimental Reproducibility | Low | Good | Good | Good |
| Cost | High | Medium | Low | Low |
| Ease of Use | + | ++ | ++ | +++ |
Applications
The unique properties of hTERT-immortalized cells, as discussed above (and illustrated in Table 1), make them an attractive replacement for both primary and transformed cell lines and a valuable tool for the study of cell functions both in vitro and in vivo. The following is a list of published applications of hTERT-immortalized cells.
- Long-term studies of biochemical and physiological aspects of cell growth, eg, endogenous protein markers, gene expression, and growth inhibition12
- In vitro model for differentiation and carcinogenesis7,12
- Cancer research and studies of oncogenes7,25
- Cell-based drug screening, and drug toxicity testing26
- Tissue engineering, and transplantation27
- Genetic engineering and modifications28
- Biological functions of hTERT29
*For karyotype information please see appendix.
ATCC hTERT-immortalized Cell Solutions
ATCC hTERT-immortalized cell lines represent a breakthrough in cell biology research that combine the in vivo nature of primary cells and the in vitro utility of continuous cell lines. Until recently, cell biologists had to choose between primary cells and established cell lines as the basis for their experimental models, but both options had their flaws. Normal primary cells are difficult to isolate, often vary from lot to lot, and senesce after a few passages. Traditional cell lines, on the other hand, are genetically unstable and present inconsistent phenotypes over time. Now, with hTERT-immortalized cell lines from ATCC, cell biologists can avoid the limitations, while enjoying the benefits of both.
In addition to the standard ATCC authentication, all ATCC hTERT cell lines are tested for:
- Extended proliferative capacity
- Stable karyotype
- Selected phenotypic markers from the tissue of interest
- Continued expression of hTERT
ATCC hTERT-cell immortalization tools
A major obstacle to the immortalization of primary human cells and the establishment of human cell lines is telomere-controlled senescence, caused by the shortening of telomeres that occurs each time somatic human cells divide.1 The enzyme telomerase can prevent the shortening of telomeres, and the transfer of exogenous hTERT cDNA (encoding the catalytic subunit of human telomerase) can be used to prevent telomere shortening, overcome telomere-controlled senescence, and immortalize primary human cells.
ATCC hTERT-immortalization vectors
This set of immortalization products enables researchers to immortalize primary cells of interest in their labs using the hTERT technology.
- hTERT vectors: ATCC MBA-141
- Viral oncoprotein vectors:
- HPV-16 E6/E7: ATCC 45113; ATCC 45113D
- Non-viral (Cdk-4 and Bmi-1) oncoprotein vectors: 81582D
ATCC hTERT-immortalized Barrett’s Esophageal Epithelial Cells
Introduction
Primary Barrett’s epithelial cells have a limited lifespan in culture and typically do not contain the genetic abnormalities that can lead to cancer in Barrett’s esophagus, which significantly limits their use as a model for the progression of this disease. In contrast, ATCC hTERT-immortalized Barrett’s esophageal epithelial cells contain stable, defined cell cycle and genetic abnormalities, have an extended life span, and are karyotypically, morphologically, and phenotypically similar to the primary parent cells.
The Barrett’s esophagus cell lines, CP-A (KR-42421), ATCC CRL-4027, CP-B (CP-52731), ATCC CRL-4028, CP-C (CP-94251), ATCC CRL-4029, and CP-D (CP-18821), ATCC CRL-4030 were derived from an endoscopic biopsy specimen obtained from a region of non-dysplastic metaplasia and transduced with the retroviral expression vector, pLXSN-hTERT.2
Cell culture protocols
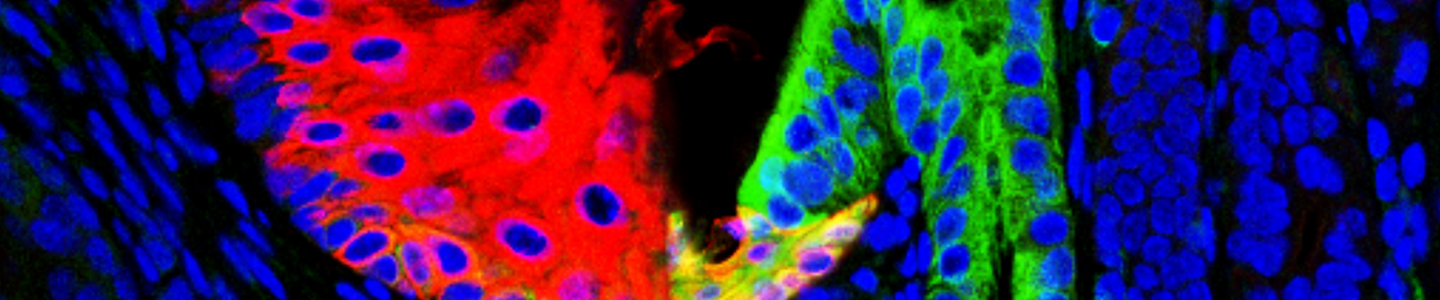
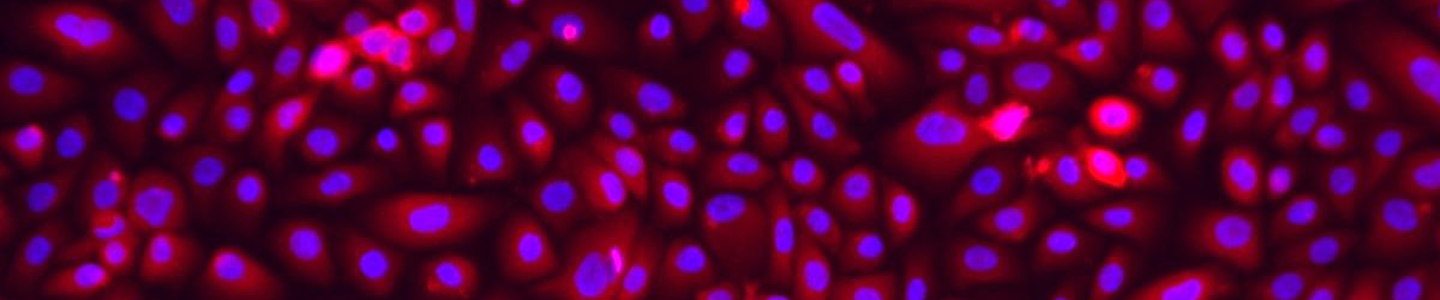
 ATCC CRL-4027 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)
ATCC CRL-4027 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)Materials needed
- MCDB-153 medium (Sigma, M7403)
- Hydrocortisone
- Recombinant human epidermal growth factor
- Cholera toxin
- Adenine
- Bovine pituitary extract
- Insulin-transferrin-sodium selenite supplement (Sigma, I1884)
- Glutamine
- Fetal bovine serum (ATCC 30-2020)
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
- Cell culture tested DMSO (ATCC 4-X)
- RPMI-1640 medium (ATCC 30-2001)
Preparation of complete growth medium
The base medium for these cell lines is MCDB-153. To make the complete growth medium add the following components to the base medium:
- 0.4 μg/mL hydrocortisone
- 20 ng/mL recombinant human epidermal growth factor
- 1 nM cholera toxin
- 20 mg/L adenine
- 140 μg/mL bovine pituitary extract
- 0.1% insulin-transferrin-sodium selenite supplement
- 4 mM glutamine
- 5% fetal bovine serum
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC primary Barrett’s epithelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Determine the number of viable cells and add an appropriate aliquot of the suspension to a culture flask.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing and maintenance of cultures
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium required proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. To facilitate dispersal of cells place the flask at 37°C.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. Proceed to the next step when the cells have reached the recommended density and are ready to be subcultured.
- Remove and discard the media.
- Rinse the cells with Dulbecco’s phosphate buffered saline to remove any trace of serum.
- Add 2.0 to 3.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: RPMI-1640 Medium supplemented with 10% fetal bovine serum and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.
ATCC hTERT-immortalized Bronchial Epithelial Cells
Introduction
The use of normal and diseased primary bronchial epithelial cultures is restricted by the limited availability of normal and diseased primary cell pairs, significant inconsistency between donors and their finite proliferative capacity. Moreover, the available cell lines have been transformed using viral genes or derived from tumors and do not maintain the morphology and phenotype of the parent cells. However, ATCC hTERT-immortalized bronchial epithelial cells have an extended lifespan, have a stable karyotype, and are phenotypically similar to the primary parent cells.
The human airway epithelial cell line, NuLi-1 (ATCC CRL- 4011), was derived from normal lung epithelial cells by dual retroviral infection with HPV-16 E6/E7-LXSN and hTERT-LXSN. Human airway epithelial cell lines, CuFi-1 (ATCC CRL-4013), CuFi-4 (ATCC CRL-4015), CuFi-5 (ATCC CRL-4016), and CuFi-6 (ATCC CRL-4017) were derived from the lung epithelial cells of cystic fibrosis patients by dual retroviral infection with HPV-16 E6/E7-LXSN and hTERT-LXSN or pBabe-hygro-hTERT.9
Cell culture protocols
 ATCC CRL-4011 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)
ATCC CRL-4011 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)Materials needed
- Bronchial Epithelial Growth Medium (BEGM), serum-free (Lonza BEGM BulletKit, CC-3170)
- G-418
- Human placental collagen type IV, (Sigma, C-7521)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
- Cell culture tested DMSO (ATCC 4-X)
- Fetal bovine serum (ATCC 30-2020)
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
Preparation of complete growth medium
The base medium for these cell lines is BEBM. To make the complete growth medium (BEGM) add the following components to the base medium:
- SingleQuot additives (supplied with the BEGM BulletKit)
- 50 µg/mL G-418
Note:
ATCC does not use the gentamycin-amphotericin B supplied with the BEGM kit.
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor phase and not at -70°C. Storage at -70°C will result in loss of viability.
The culture flasks should be pre-coated with 60 µg/mL solution of human placental collagen type IV at least 18 hours in advance, then air-dried and rinsed 2 to 3 times with Dulbecco’s phosphate buffered saline.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC primary bronchial epithelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents, the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing procedure
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
The culture flasks should be pre-coated with 60 µg/mL solution of human placental collagen type IV at least 18 hours in advance, then air-dried and rinsed 2 to 3 times with Dulbecco’s phosphate buffered saline.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days (do not exceed 3 days).
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Add 2.0 to 3.0 mL trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- To remove trypsin-EDTA solution, add 2.0 to 3.0 mL of 1% FBS in Dulbecco’s phosphate buffered saline and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
Cryopreservation medium
Freeze cells in the following medium: BEGM supplemented with 10% DMSO and 30% fetal bovine serum. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.

ATCC hTERT-immortalized Chondrocyte Fibroblast Cells
Introduction
Primary human fetal chondrocytes exhibit a relatively short life span under standard culture conditions, which severely restricts their ability to be used in research studies. ATCC hTERT-immortalized chondrocytes have an extended lifespan, retain expression of chondrocyte specific markers, and are karyotypically, morphologically, and phenotypically similar to the primary parent cells.
The chondrocyte cell lines, CHON-001 (ATCC CRL-2846) and CHON-002 (ATCC CRL-2847), were derived from chondrocytes of normal human long bones infected by hTERT-LXSN under G-418 selection.
Cell culture protocols
Materials needed
- ATCC-formulated Dulbecco’s modified Eagle’s medium (DMEM) (ATCC 30-2002)
- G-418
- Fetal bovine serum (ATCC 30-2020)
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
- Trypsin-EDTA Solution (0.05% Trypsin/0.53 mM EDTA, such as Gibco 25300-054) or Trypsin-EDTA for primary cells (ATCC PCS-999-003)
- Cell culture tested DMSO (ATCC 4-X)
Preparation of complete growth medium
The base medium for these cell lines is ATCC-formulated DMEM. To make the complete growth medium, add the following components to the base medium:
- 0.1 mg/mL G-418
- 10% heat-inactivated fetal bovine serum
Note:
This medium is formulated for use with a 5% CO2 in air atmosphere. (Standard DMEM formulations contain 3.7 g/L sodium bicarbonate and a 10% CO2 in air atmosphere is then recommended).
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC primary human fetal chondrocytes.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents, the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing procedure
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 days, or as needed.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Rinse the cells with Dulbecco’s phosphate buffered saline to remove traces of serum.
- Add 2.0 to 3.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- To remove trypsin-EDTA solution, add 2.0 to 3.0 mL of 1% FBS in Dulbecco’s phosphate buffered saline and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: 90% heat-inactivated fetal bovine serum and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.

ATCC hTERT-immortalized Dermal Microvascular Endothelial Cells
Introduction
ATCC hTERT-immortalized dermal microvascular endothelial cells have an extended lifespan, express a panel of endothelial cell surface proteins, undergo tubule formation in culture, and are karyotypically, morphologically, and phenotypically similar to the primary parent cells. By overcoming the finite proliferative capacity of primary cells, the hTERT dermal microvascular endothelial cells represent an effective cell model for studying endothelial cell biology including signal transduction and angiogenesis.
The telomerase-immortalized human microvascular endothelium cell line, TIME (ATCC CRL-4025), was derived from a primary culture of neonatal foreskin microvascular endothelial cells (HMVEC) of the dermis. Primary HMVECs were immortalized by infection with the retrovirus WZLblast3:hTERT. TIME cells express a panel of characteristic endothelial cell surface marker proteins including CD31/PECAM-1 and integrin αVβ3. The cells also express the low density lipoprotein (LDL) receptor and are capable of acetylated LDL uptake.12
Cell culture protocols
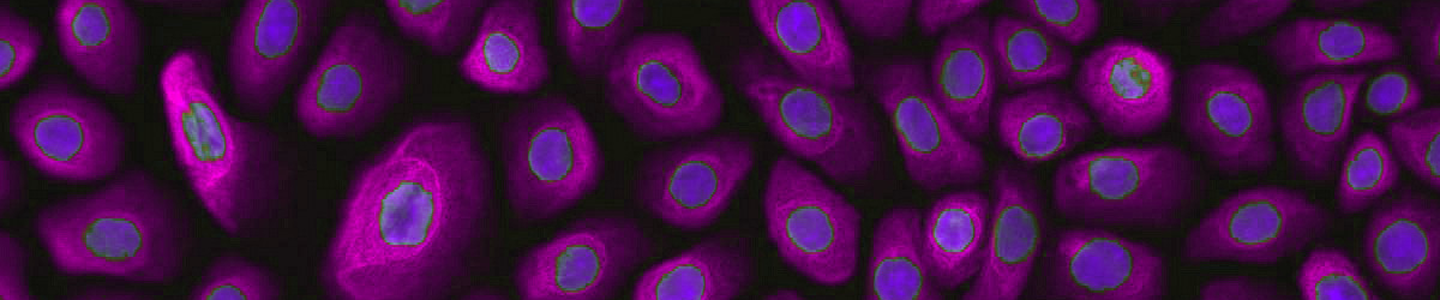
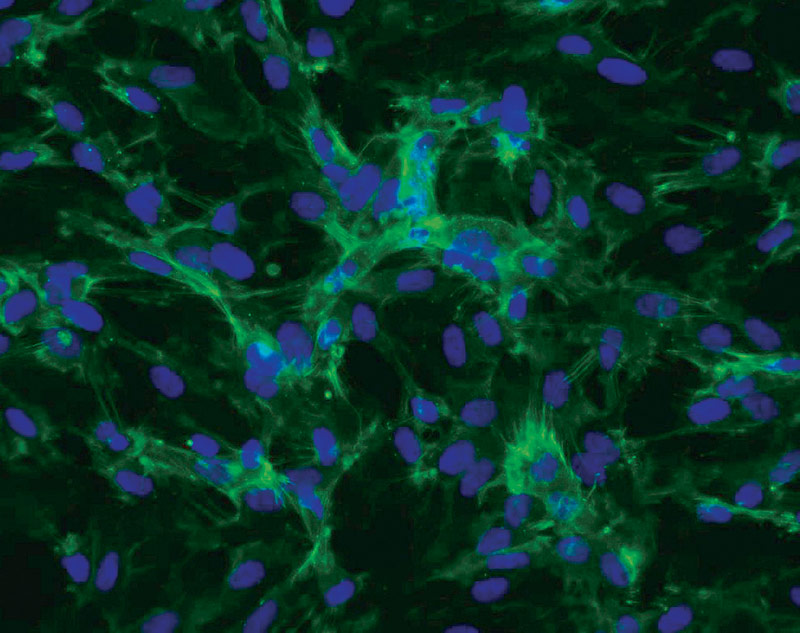 ATCC CRL-4025 stained with a CD31 antibody (green) and Hoechst dye (blue)
ATCC CRL-4025 stained with a CD31 antibody (green) and Hoechst dye (blue)Materials needed
- Endothelial Cell Basal Medium-2 (EBM-2), (Lonza EGM-2-MV BulletKit, CC-3202)
- Blasticidine
- Trypsin-EDTA solution (Lonza, CC-5012)
- Trypsin neutralizing solution (Lonza, CC-5002)
- Fetal bovine serum (ATCC 30-2020)
- HEPES buffered saline solution (Lonza, CC-5024)
- Cell culture tested DMSO (ATCC 4-X)
Preparation of complete growth medium
The base medium for these cell lines is Endothelial Cell Basal Medium-2 (EBM-2). To make the complete growth medium (EGM-2), add the following components to the base medium:
- SingleQuot additives supplied with the kit
- 12.5 µg/mL blasticidine
Note:
ATCC does not use the gentamycin-amphotericin B mix supplied with the kit.
Note:
Do not filter complete medium.
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor phase and not at -70°C. Storage at –70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC dermal microvascular endothelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents, the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing procedure
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made, proceed to the next step.
- Remove and discard the media.
- Rinse the cells with room temperature HEPES buffered saline solution.
- Add 5.0 to 6.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 4 to 8 minutes).
- To inactivate trypsin-EDTA solution, add 5.0 to 6.0 mL of trypsin neutralizing solution and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: 90% fetal bovine serum and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.

ATCC hTERT-immortalized Endometrial Fibroblasts
Introduction
ATCC hTERT-immortalized endometrial fibroblasts (T HESCs) have an extended lifespan and are karyotypically, morphologically, and phenotypically similar to the primary parent cells. Functionally, T HESCs display the biochemical endpoints of decidualization after hormone treatment.
T HESCs (ATCC CRL-4003) were derived from stromal cells obtained from an adult female with myomas. The primary stromal endometrium cells were immortalized by infection with supernatant from the packaging cell line pA317-hTERT which expressed the hTERT and the puromycin resistance genes.23
Cell culture protocols
Materials needed
- Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Sigma, D2906)
- Sodium bicarbonate
- ITS+ Universal Culture Supplement Premix (BD, 354352)
- Puromycin
- Charcoal/dextran treated fetal bovine serum (Hyclone, SH30068.03)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
- Cell culture tested DMSO (ATCC 4-X)
Preparation of complete growth medium
The base medium for these cell lines is DMEM/F12 supplemented with:
- 1.5 g/L sodium bicarbonate
- 1% ITS+ Universal Culture Supplement Premix
- 500 ng/mL puromycin
- 10% charcoal/dextran treated fetal bovine serum
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC endometrial fibroblasts cells.
- Prepare a 25 cm² or a 75 cm² culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents, the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed, and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 6.0 to 8.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing procedure
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not hit or shake the flask while waiting for the cells to detach. Cells that are difficult to detach may be placed at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made, proceed to the next step.
- Remove and discard the media.
- Add 2.0 to 3.0 mL of Trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 15 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels.
Refer to the product sheet for the recommended inoculum concentration.
Cryopreservation medium
Freeze cells in the following medium: 95% complete growth media, 5% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.
ATCC hTERT-immortalized Human Foreskin Fibroblasts
Introduction
Human foreskin fibroblasts were among the first primary cell types to be successfully immortalized using hTERT technology, so the functions and properties of hTERT-immortalized fibroblasts have been thoroughly characterized. ATCC hTERT-immortalized foreskin fibroblasts (BJ-5ta) have an extended lifespan, express PDGFRβ, and are karyotypically, morphologically, and phenotypically similar to the primary parent cells. BJ 5ta are widely used as in vitro models for wound healing, tissue engineering, and regeneration applications.
The hTERT-immortalized foreskin fibroblast cell line, BJ-5ta (ATCC CRL-4001), was derived by transfecting the BJ foreskin fibroblast cell line, at population doubling (PDL) 58, with pGRN145 hTERT followed by selection with hygromycin B.25
Cell culture protocols
Materials needed
- Dulbecco’s Modified Eagle’s Medium (DMEM) (ATCC 30-2002)
- M199 medium
- Hygromycin B
- Fetal bovine serum
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
Preparation of complete growth medium
The base medium for this cell line is a 4:1 mixture of DMEM and M199. To make the complete growth medium add the following components to the base medium:
- 0.01 mg/mL hygromycin B
- 10% fetal bovine serum
 Handling procedure for frozen cells and initiation of cultures
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of human foreskin fibroblasts.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing and maintenance of cultures
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Rinse the cells with Dulbecco’s phosphate buffered saline to remove traces of serum.
- Add 2.0 to 3.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate the cultures in a 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: 90% fetal bovine serum and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.

ATCC hTERT-immortalized Mammary Epithelial Cells
Introduction
ATCC hTERT-immortalized mammary epithelial cells (hTERT-HME1) have an extended lifespan, do not express estrogen receptor, and are karyotypically, morphologically, and phenotypically similar to the primary parent cells. In addition, these cells retain many differentiated features of normal HMECs. As such, hTERT-HME1 can serve as valuable in vitro models to study the stages of breast cancer development.
The human mammary epithelium, HME1 (ATCC CRL-4010) cell line was derived from normal primary mammary epithelial cells infected with the retrovirus pBabepuro+hTERT vector and cultured in complete growth medium containing puromycin until stable clones were selected.13
Cell culture protocols
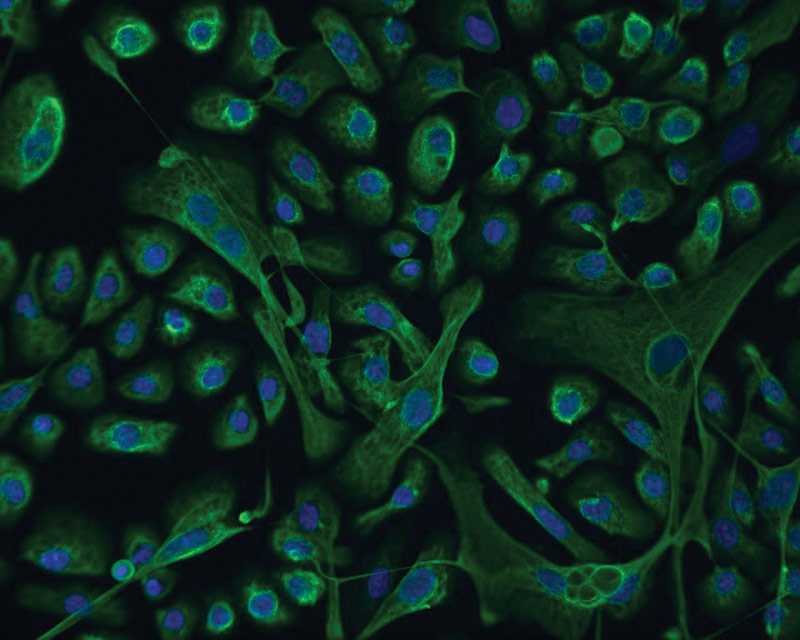
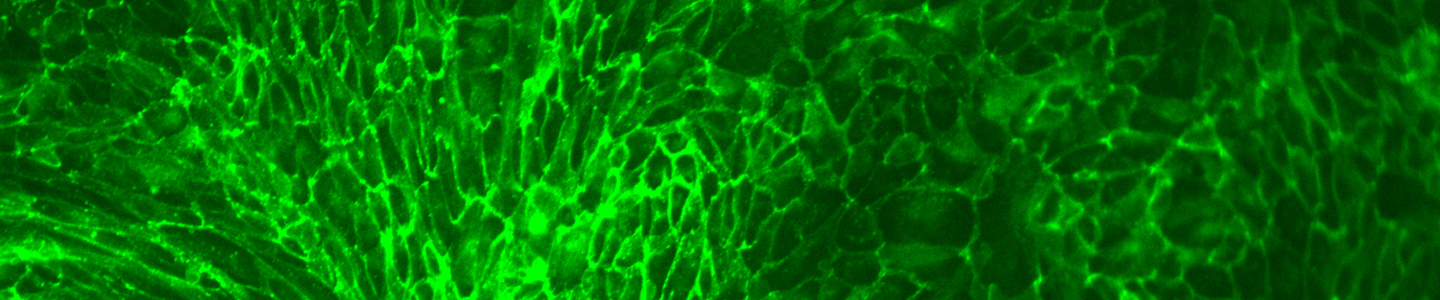
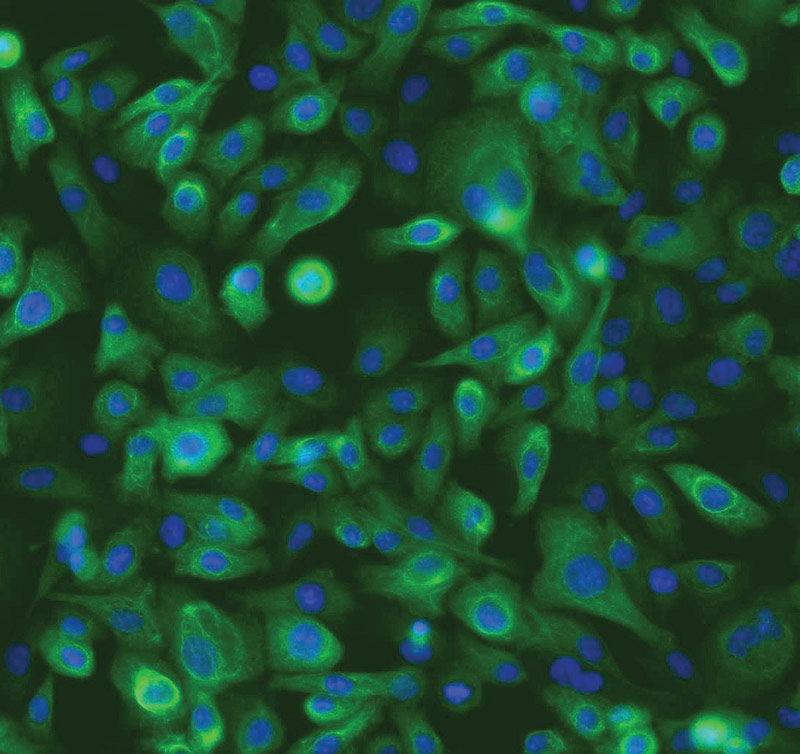 ATCC CRL-4010 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)
ATCC CRL-4010 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)Materials needed
- Mammary Epithelial Growth Medium, serum-free (MEGM) from Clonetics (Lonza MEGM BulletKit, CC-3150)
- Soybean trypsin inhibitor (ATCC 30-2104)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
Preparation of complete growth medium
The base medium for these cell lines is MEBM. To make the complete growth medium (MEGM) add the following components to the base medium:
- SingleQuot additives (supplied with the MEGM BulletKit)
Note:
ATCC does not use the gentamycin- amphotericin B mix provided with the kit. Do not filter complete medium.
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC immortalized mammary epithelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing and maintenance of cultures
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Add 2.0 to 3.0 mL of soybean trypsin inhibitor solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
Cryopreservation medium
Freeze cells in the following medium: 90% complete culture medium supplemented and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.

ATCC hTERT-immortalized Pancreas Duct Epithelial Cells
Introduction
Investigating the roles played by cells expressing the neuronal stem cell marker nestin in the human pancreas is important in pancreatic cancer and metabolic disease studies. ATCC hTERT-immortalized pancreas duct epithelial cells have an extended lifespan, express nestin, and are karyotypically, morphologically, and phenotypically similar to the primary parent cells.
hTERT-HPNE (Human Pancreatic Nestin Expressing cells) (ATCC CRL-4023) was developed from human pancreatic duct cells by transduction with a retroviral expression vector (pBABEpuro) containing the hTERT gene. hTERT-HPNE E6/E7 (ATCC CRL- 4036) cells were derived from hTERT-HPNE cells (ATCC CRL- 4023) by infection with retroviral vector pLEXSN carrying HPV-16 E6/E7. hTERT-HPNE E6/E7/st cells were derived from hTERT-HPNE E6/E7 cells (ATCC CRL-4036) by infection with retroviral vector pBabeZeo carrying the SV40 small T-antigen. hTERT-HPNE E6/E7/K-RasG12D cells were derived from hTERT-HPNE E6/E7 cells (ATCC CRL-4036) by infection with retroviral vector pLXSN carrying a G12D mutant of the isoform b of human K-Ras. hTERT-HPNE E6/E7/K-RasG12D/st cells were derived from hTERT-HPNE E6/E7/K-RasG12D cells (ATCC CRL-4038) by infection with retroviral vector pBabeZeo carrying the SV40 small T-antigen.7
Cell culture protocols
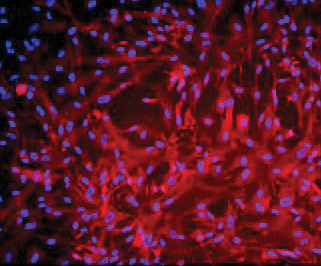
ATCC CRL-4023 stained with a monoclonal pan-cytokeratin antibody (red) and Hoechst dye (blue)
Materials needed
- DMEM without glucose (Sigma D-5030 with additional 2 mM L-glutamine and 1.5 g/L sodium bicarbonate)
- Medium M3 Base (Incell Corp. M300F-500)
- Recombinant human epidermal growth factor
- D-glucose
- Puromycin
- Cell culture tested DMSO (ATCC 4-X)
- Fetal bovine serum (ATCC 30-2020)
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
Preparation of complete growth medium
The base medium for this cell line is:
- 75% DMEM without glucose
- 25% Medium M3 Base
To make the complete growth medium, add the following components to the base medium:
- Fetal bovine serum to a final concentration of 5%
- 10 ng/mL human recombinant EGF
- 5.5 mM D-glucose (1g/L)
- 750 ng/mL puromycin
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC pancreas duct epithelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing and maintenance of cultures
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Rinse the cells with Dulbecco’s phosphate buffered saline.
- Add 2.0 to 3.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate cultures in a 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: 90% fetal bovine serum and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.
ATCC hTERT-immortalized Renal Epithelial Cells
Introduction
Angiomyolipomas are benign tumors of the kidney, which originate from putative perivascular epithelioid cells. These cells may undergo differentiation into cells with features of melanocytes, smooth muscle, or fat cells. However, the study of angiomyolipomas is limited by a lack of established angiomyolipoma-derived cell lines and a lack of good animal models. ATCC hTERT-immortalized renal epithelial cells are derived from angiomyolipomas, so researchers can now take the advantage of both the primary cell characteristics and extended lifespan of these cells in vitro.
The UMB1949 cell line (ATCC CRL-4004) expresses NG2 and L1 and has a defined 5bp deletion in exon 33 of tuberin (Tsc2) and mutations in tuberin (and/or hamartin). As such, this cell line can be used to study signal transduction and drug efficiency in tuberous sclerosis. The SV7tert PDGFtu1 (ATCC CRL-4008) cell line is derived from the tumors caused by the SV7tert implantation in nude mice. The SV7tert cell line is a non-tumorigenic angiomyolipoma cell line immortalized with the SV40 large T antigen and human telomerase, by transduction with a retrovirus encoding PDGF-BB. The tumor-derived cells secrete over 18-fold more PDGF than pre-implantation cells, and demonstrate both autocrine transformation and epigenetic changes.5
Cell culture protocols
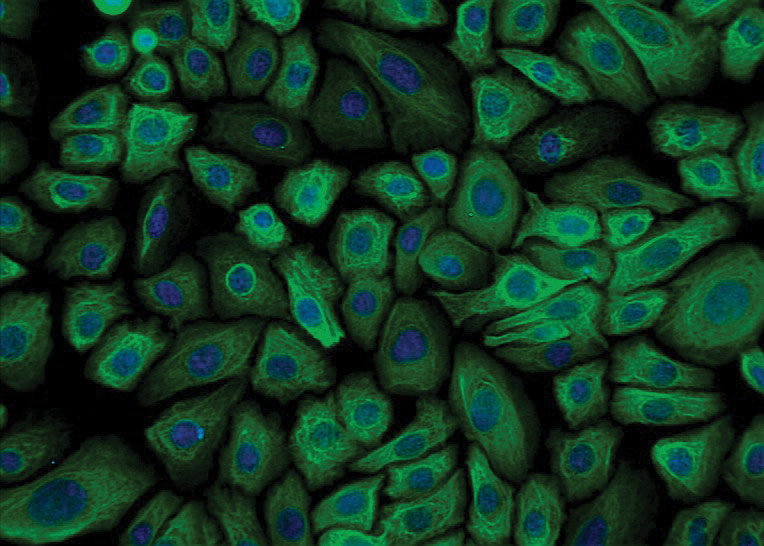
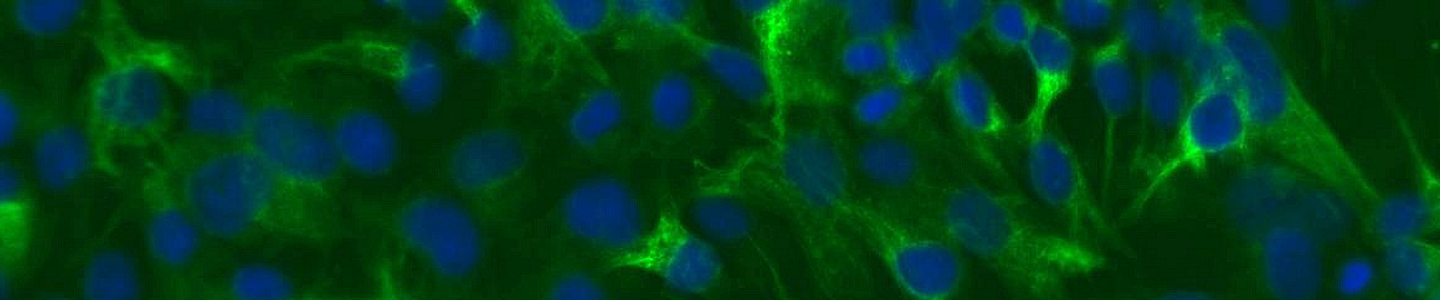
 ATCC CRL-4004 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)
ATCC CRL-4004 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)Materials needed
- Dulbecco’s Modified Eagle’s Medium (DMEM) (ATCC 30-2002)
- Fetal bovine serum (ATCC 30-2020)
- Dulbecco’s phosphate buffered saline (ATCC 30-2200)
- Trypsin-EDTA Solution (0.25% Trypsin/0.53 mM EDTA in HBSS) (ATCC 30-2101)
- Cell culture tested DMSO (ATCC 4-X)
Preparation of complete growth medium
The base medium for these cell lines is DMEM. To make the complete growth medium add the following components to the base medium:
- Fetal bovine serum to a final concentration of 10%
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC renal epithelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing and maintenance of cultures
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Rinse the cells with Dulbecco’s Phosphate Buffered Saline to remove traces of serum.
- Add 2.0 to 3.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate the cultures in a 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: 95% complete growth medium, 5% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.
ATCC hTERT-immortalized Retinal Pigmented Epithelial Cells
Introduction
Retinal pigment epithelium (RPE) is the layer of cells at the base of the retina composed of a single layer of hexagonal cells that are densely packed with pigment granules. The RPE protects and supplies nutrition to the retina, and degeneration of the RPE is linked to age-related macular degeneration (AMD). The hTERT-immortalized RPE cell line is a valuable tool in AMD research, with application to studies of RPE functions, disease progression, RPE regeneration, and RPE wound healing.
The hTERT-immortalized retinal pigmented epithelial cell line hTERT RPE-1 (ATCC CRL-4000) was derived by transfecting the RPE-340 cell line with the pGRN145 hTERT-expressing plasmid (ATCC MBA-141). Cells were cultured in medium containing hygromycin B until stable clones were selected.11
Cell culture protocols
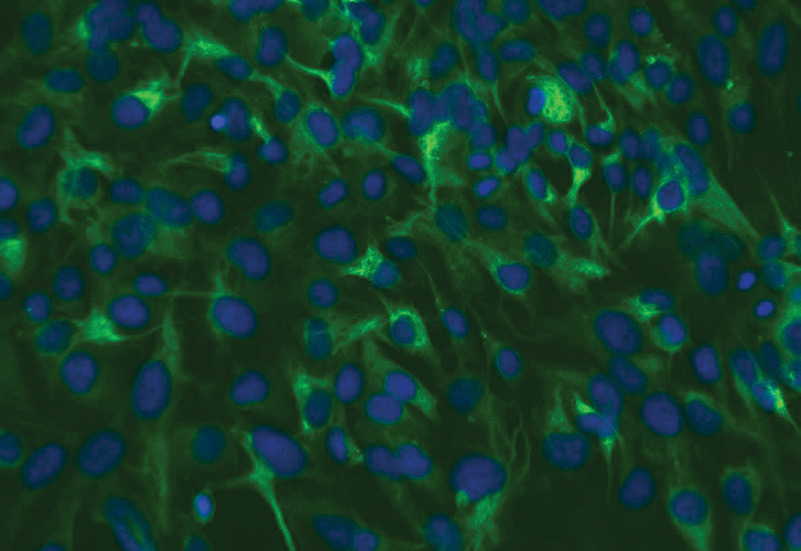
 ATCC CRL-4000 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)
ATCC CRL-4000 stained with a monoclonal pan-cytokeratin antibody (green) and Hoechst dye (blue)Materials needed
- Dulbecco’s Modified Eagle’s Medium (DMEM)/ F12 (ATCC 30-2006)
- Fetal bovine serum (ATCC 30-2020)
- Hygromycin B
- Hank's balanced salt solution (ATCC 30-2213)
- Trypsin-EDTA Solution (ATCC 30-2101)
- Cell culture tested DMSO (ATCC 4-X)
Preparation of complete growth medium
The base medium for this cell line is ATCC-formulated DMEM: F12. To make the complete growth medium, add the following components to the base medium:
- Fetal bovine serum to a final concentration of 10%
- 0.01 mg/mL hygromycin B
Handling procedure for frozen cells and initiation of cultures
To ensure the highest level of viability, thaw the vial and initiate the culture as soon as possible upon receipt. If storage of the frozen culture is necessary upon arrival, store the vial in liquid nitrogen vapor and not at -70°C. Storage at -70°C will result in loss of viability.
- Refer to the batch specific information provided on the last page of the product information sheet for the total number of viable cells recovered from each lot of ATCC retinal pigmented epithelial cells.
- Prepare a culture flask containing the recommended complete culture medium. Prior to the addition of the vial contents the vessel containing the growth medium should be placed in the incubator for at least 15 minutes to allow the medium to reach its normal pH (7.0 to 7.6) and to avoid excessive alkalinity of the medium during recovery of the cells.
- Thaw the vial by gentle agitation in a 37°C water bath. To reduce the possibility of contamination, keep the O-ring and cap out of the water. Thawing should be rapid (approximately 2 minutes).
- Remove the vial from the water bath as soon as the contents are thawed and decontaminate by dipping in or spraying with 70% ethanol. All operations from this point on should be carried out under strict aseptic conditions.
- Transfer the vial contents to a centrifuge tube containing 9.0 mL of complete culture medium and centrifuge the cell suspension at approximately 125 x g for 5 to 7 minutes.
- Discard the supernatant and resuspend the cells in fresh growth medium (see the batch-specific information for the recommended dilution ratio). Add this suspension to the prepared culture vessel.
- Incubate the culture in a 37°C, 5% CO2, humidified incubator.
Subculturing and maintenance of cultures
Volumes are given for a 75 cm2 flask. Increase or decrease the amount of dissociation medium needed proportionally for culture vessels of other sizes.
Note:
To avoid clumping do not agitate the cells by hitting or shaking the flask while waiting for the cells to detach. Place at 37°C to facilitate dispersal.
- Maintain the cells in culture by refreshing the medium every 2 to 3 days.
- Refer to the product information sheet for the concentration at which cells should be subcultured. When a determination that the cells are ready to be subcultured is made proceed to the next step.
- Remove and discard the media.
- Rinse with Hank's balanced salt solution to remove traces of serum.
- Add 2.0 to 3.0 mL of trypsin-EDTA solution to the flask and observe cells under an inverted microscope until the cell layer is dispersed (usually within 5 to 10 minutes).
- Add 6.0 to 8.0 mL of complete growth medium and aspirate cells by gently pipetting.
- Transfer cell suspension to a 15 mL centrifuge tube and spin at approximately 125 x g for 5 to 10 minutes.
- Discard supernatant and resuspend cells in fresh growth medium. Add appropriate aliquots of the cell suspension to new culture vessels. Refer to the product sheet for the recommended inoculum concentration.
- Incubate cultures at 37°C, 5% CO2, humidified incubator.
Cryopreservation medium
Freeze cells in the following medium: 30% culture medium, 60% fetal bovine serum, and 10% DMSO. Store vials in liquid nitrogen vapor. Avoid immersing vials into liquid nitrogen.
Download a PDF of our hTERT-immortalized cell culture guide
Download Now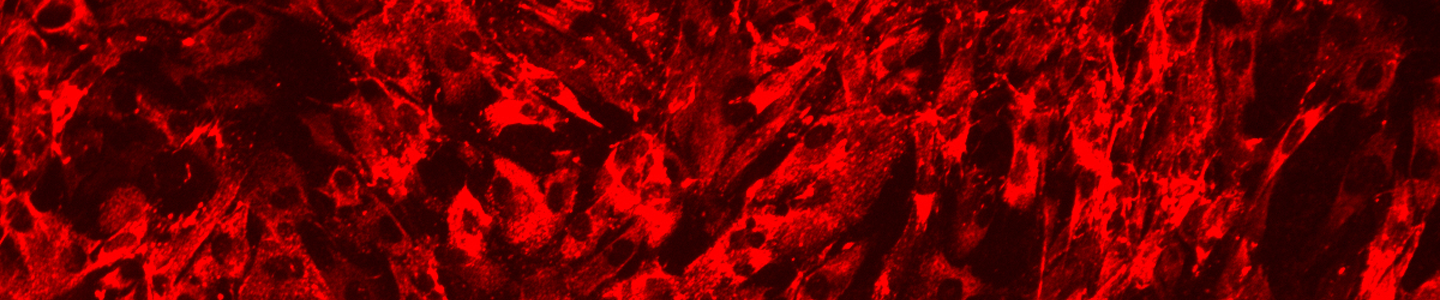
Appendix
hTERT karyotype information
| ATCC number | Karyotype |
|---|---|
| CRL-2846 | This is a diploid cell line of female origin. Overall, the karyology is stable with a modal chromosome number of 46 in 93% of the examined cells and a low rate of polypoidy. No consistent structural chromosomal aberrations were found in any of the cells examined. |
| CRL-2847 | This is a diploid cell line of female origin. Overall, the karyology is stable with a modal chromosome number of 46 in 87% of the examined cells and a low rate of polypoidy. No consistent structural chromosomal aberrations were found in any of the cells examined. |
| CRL-4000 | This is a near-diploid human cell line of female origin with a modal chromosome number of 46 that occurred in 90% of the cells counted. The sex chromosomes consist of a karyotypically normal X-chromosome and a derivative X-chromosome with additional chromosomal material at the terminal end of the q-arm. The derivative X-chromosome was present in all of the cells analyzed. |
| CRL-4001 | This is a diploid human cell line of male origin with a modal chromosome number of 46 that occurred in 90% of the cells counted. The sex chromosomes, X and Y are both karyotypically normal. |
| CRL-4003 | This is a diploid cell line of female origin. Overall, the karyology is stable with a modal chromosome number of 46 in 57% of the examined cells and a low rate of polyploidy. No consistent structural chromosomal aberrations were found in any of the cells examined. |
| CRL-4004 | This cell line is of male origin and 1/2 to 2/3 of the total cell population is pseudo-diploid, the rest of the cells fall in the tetraploid range. Consistent cytogenetic changes include chromosome 10 and 19 aberration, and chromosome 4 monosomy. Some cells showed loss of the Y chromosome and many of the examined cells contained random chromosomal aberrations. |
| CRL-4008 | This is a hypotetraploid cell line with many structural rearrangements, numerical losses, and gains. The following eight derivatives were found to be present in low and high passage karyotypes: der(x)t(X;3)(q28;p21), der(1)t(1;17) (q10;p10), der(3)t(3;6)(p10:p10), i(8)(q10), i(12)(q10), der(13)t(13;21)(q10;q10), der(16)t(4;16)(q21;q24), add(20) (q13.3). Generally, the karyotyped passages contained the same complement of chromosome rearrangements, losses, and gains. |
| CRL-4010 | This is a pseudo-diploid cell line of female origin with a modal chromosome count of 46 and a low-to-moderate rate of polyploidy. However, even though the line generally has 46 chromosomes per cell, several of those 46 were derivative or marker chromosomes. There were 2 copies of a karyotypically normal X-chromosome present in 50-60% of the cells. Other features included a normal variation in the heterochromatic region of chromosome 1 (1qh+), a consistent derivative-10 marker chromosome (present in most cells), and 2 other markers: del(3)(p24?) and del(16)(q21~23?) (present in approximately 20-30% of the analyzed cells). Overall, approximately 3-8 marker chromosomes were present in the analyzed metaphase spreads and satellite associations appeared sporadically. |
| CRL-4011 | This is a near-diploid human cell line of male origin with a polyploidy rate of 24%. There were copies of karyotypically normal X and Y-chromosomes present in most of the cells analyzed. Overall, some of the cells contained chromosomal abnormalities, with the most consistent being trisomy 5 and 20. |
| CRL-4013 | This is a near-diploid human cell line of female origin with a modal chromosome count of 46 and a polyploidy rate of 27%. There were 2 copies of a karyotypically normal X-chromosome present in most of the cells. Overall, some of the cells contained chromosomal abnormalities, with most consistent being trisomy 20. |
| CRL-4015 | The karyotypes of several different passages were determined. This is a human cell line of female origin, and the ploidies range from near-diploid to near-tetraploid. The karyology seems to stabilize at higher passages in the hyperdiploid range with trisomies or tetrasomies of chromosomes 1, 5, 8, 11, and 20. Additional copies of chromosomes 5 and 20 were the most consistent aberrations found throughout all the passages and ploidies. |
| CRL-4016 | This is a near-diploid cell line of male origin in which the most consistent karyotypic aberrations are trisomy of chromosomes 5 and 20. Other non-clonal aberrations were found at early passage, but the karyology tended to stabilize within several passages. |
| CRL-4017 | This is a near-diploid cell line of male origin. The most consistent karyotypic aberrations include trisomy of chromosome 5 and 20, monosomy of chromosome 15 or 16, and loss of the Y chromosome. Additionally, the polyploidy rate may increase slightly at high passage. |
| CRL-4023 | This is a pseudo-diploid human cell line of male origin with a modal chromosome number of 46 and a low polyploidy rate. Approximately 50% of the cells contained a consistent derivative chromosome 21 with additional material at p12. |
| CRL-4025 | This is a diploid cell line of male origin with a modal chromosome number of 46 and a low rate of polyploidy. The line shows some karyotypic instability at later passages. |
| CRL-4027 | This is a near-diploid cell line of male origin in which 2 sub-clones make up the majority of the cell population. One clone containing i(8)(q10) and trisomy 20 and the other containing der(1)t(1;18)(q10;q10), i(8)(q10), der(13)t(13;22) (q10;q10) and trisomy 20. The remaining population is generally made up of cells with non-clonal aberrations that were derived from the 2 major clones. Also, the non-clonal cell population may increase at high passages. |
| CRL-4028 | This is a hypodiploid cell line of male origin with the following derivative chromosomes consistently present at different passages: der(1)t(1;17)(q42?;q21), add(8)(p11.2), der(9)t(9;14)(q10;q10), add(12)(q13), add(15)(q24.3), add(17)(p11.2), del(19)(p13.1), del(21)(q22.1). It should be noted that the tetraploid population was essentially a duplicate of the hypodiploid population and may range from around 18% at lower passages to as high as 50% at higher passages. |
| CRL-4029 | Genetic instability studies using flow cytometry and FISH reveal the retention of elevated tetraploidy (G2/ tetraploidy) in the hTERT-immortalized cells similar to the non-transduced parental cells. |
| CRL-4030 | This is a hypotetraploid human cell line with the following derivative chromosomes consistently present at several different passages: add(2)(q13), der(3)t(3;8)(p10;q10), ider(7)(q10)dup(q31), der(12)t(12;13)(p10;q10), der(14) t(14;15)(q10;q10), der(15)t(15;22)(q10;q10), add(22)(q13)x2. In addition, there were consistent losses of one copy of chromosomes X, 10, 13, 14, 15, 19, and 20. Other less consistent structural aberrations were observed in some of the examined cells. |
| CRL-4036 | This is a cell line of male origin that contains 2 major clonal cell populations: 45~47, XY, der(21)t(17;21)(q21.3;p13) and 46,XY, t(3;18) (p21.1;q11.2),der(21)t(17;21)(q21.3;p13). Other chromosomal aberrations were observed in the examined cells of both clones, but none were of a consistent nature. |
| CRL-4037 | This is a pseudo-diploid human cell line of male origin with a der(21)t(17;21)(q21.3;p13). Another subclone, present at earlier passages, may contain the additional derivative chromosome: der(3)t(3;18)(p21.1;q11.2). Overall, the cell line has a relatively stable karyotype. |
| CRL-4038 | This is a pseudo-diploid human cell line of male origin. Clonal aberrations included the derivative chromosomes: t(3;18)(p21;q11.2) [balanced translocation], del(6)(q15), add(8)(q11.2) and der(21)t(17;21)(q21;p11.2). The percentage of cells with the normal male chromosome complement increased at high passage and non-clonal aberrations were seen in approximately 20% of the examined cells at all passages. |
| CRL-4039 | This is a human cell line of male origin with three major clonal cell populations: 45~48,XY,t(3;18)(p21;q11.2),der(21) t(17;21) (q21;p11.2), 47~48,idem,+20 and 45~48,XY, der(21)t(17;21)(q21;p11.2). Other non-clonal, chromosomal aberrations may be present in the cells of the three major clones. |
Tissue Types
| Tissue type | hTERT-immortalized cells | ATCC number | Designations |
|---|---|---|---|
| Adipose tissue | hTERT-immortalized adipose-derived mesenchymal stem cells (MSC) | SCRC-4000 | ASC52telo |
| Adipose tissue | hTERT A41hWAT-SVF superficial neck fat; adipose derived, fibroblast like | CRL-3386 | hTERT A41hWAT-SVF |
| Breast | hTERT human mammary epithelium, normal | CRL-4010 | hTERT-HME1(ME16C) |
| Bone | hTERT human bone cartilage fibroblast, normal | CRL-2846 | CHON-001 |
| Bone | hTERT human bone cartilage fibroblast, normal | CRL-2847 | CHON-002 |
| Esophagus | hTERT human Barrett’s esophageal epithelium | CRL-4027 | CP-A (KR-42421) |
| Esophagus | hTERT human Barrett’s esophageal epithelium | CRL-4028 | CP-B (CP-52731) |
| Esophagus | hTERT human Barrett’s esophageal epithelium | CRL-4029 | CP-C (CP-94251) |
| Esophagus | hTERT human Barrett’s esophageal epithelium | CRL-4030 | CP-D (CP-18821) |
| Eye | hTERT retinal pigmented epithelial cells, normal | CRL-4000 | hTERT RPE-1 |
| Fallopian Tube | hTERT-immortalized human fallopian tubule cells, preB-ALL, relapse | CRL-3445 | hTERT FT 194 |
| Gingiva | hTERT TIGKs gingival epithelium | CRL-3397 | hTERT TIGKs |
| Gingiva | hTERT-immortalized human gingival fibroblast, normal, adult | CRL-4061 | hTERT Gingival Fibroblast |
| Heart | hTERT human aortic endothelium, normal | CRL-4052 | TeloHAEC |
| Heart | hTERT human aortic endothelium, normal | CRL-4054 | TeloHAEC-GFP |
| Kidney | hTERT human renal epithelium, tuberous sclerosis, angiomyolipoma | CRL-4004 | UMB1949 |
| Kidney | hTERT human renal epithelium, tuberous sclerosis, angiomyolipoma | CRL-4008 | SV7tert PDGF tumor-1 |
| Kidney | hTERT human renal proximal tubule epithelium | CRL-4031 | RPTEC/TERT1 |
| Kidney | hTERT human renal proximal tubule epithelium | CRL-4031-OAT1 | RPTEC/TERT1 OAT1 |
| Kidney | hTERT human renal proximal tubule epithelium | CRL-4031-OCT2 | RPTEC/TERT1 OCT2 |
| Kidney | hTERT human renal proximal tubule epithelium | CRL-4031-OAT3 | RPTEC/TERT1 OAT3 |
| Lung | hTERT human bronchial epithelium, normal | CRL-4011 | NuLi-1 |
| Lung | hTERT human bronchial epithelium, cystic fibrosis | CRL-4013 | CuFi-1 |
| Lung | hTERT human bronchial epithelium, cystic fibrosis | CRL-4015 | CuFi-4 |
| Lung | hTERT human bronchial epithelium, cystic fibrosis | CRL-4016 | CuFi-5 |
| Lung | hTERT human bronchial epithelium, cystic fibrosis | CRL-4017 | CuFi-6 |
| Lung | hTERT human small airway epithelium, normal | CRL-4050 | HSAEC1-KT |
| Lung | hTERT human bronchial epithelium, normal | CRL-4051 | HBEC3-KT |
| Lung | hTERT lung fibroblast | CRL-4058 | hTERT Lung Fibroblast |
| Neural | hTERT ipNF05.5 (Mixed clones) human plexiform neurofibroma | CRL-3387 | hTERT NF1 ipNF05.5 (Mixed clones) |
| Neural | hTERT ipNF05.5 human plexiform neurofibroma | CRL-3388 | hTERT NF1 ipNF05.5 |
| Neural | hTERT ipNF95.6 human plexiform neurofibroma | CRL-3389 | hTERT NF1 ipNF95.6 |
| Neural | hTERT ipNF95.11b human C plexiform neurofibroma | CRL-3390 | hTERT NF1 ipNF95.11b C |
| Neural | hTERT ipNF95.11c human Schwann cell | CRL-3391 | hTERT NF1 ipnNF95.11c |
| Neural | hTERT ipn02.3 2λ human Schwann cell | CRL-3392 | hTERT NF1 ipn02.3 2λ |
| Pancreas | hTERT human normal pancreatic duct epithelium, normal | CRL-4023 | hTERT-HPNE |
| Pancreas | hTERT human pancreatic duct epithelium | CRL-4036 | hTERT-HPNE E6/E7 |
| Pancreas | hTERT human pancreatic duct epithelium | CRL-4037 | hTERT-HPNE E6/E7/st |
| Pancreas | hTERT human pancreatic duct epithelium | CRL-4038 | hTERT-HPNE E6/E7/K- RasG12D |
| Pancreas | hTERT human pancreatic duct epithelium | CRL-4039 | hTERT-HPNE E6/E7/K- RasG12D/st |
| Prostate | hTERT human prostate epithelium, normal | CRL-3289 | hTERT EP156T |
| Prostate | hTERT human prostate fibroblast, cancer associated | CRL-3290 | hTERT PF179T CAF |
| Prostate | hTERT human prostate fibroblast, normal | CRL-3291 | hTERT SMC PM151T |
| Skin | hTERT human foreskin fibroblast, normal | CRL-4001 | BJ-5ta |
| Skin | hTERT human skin fibroblast, cerebro-oculo-facio-skeletal-syndrome | CRL-4005 | TelCOFS02MA |
| Skin | hTERT human dermal microvascular endothelium, normal | CRL-4025 | TIME |
| Skin | hTERT human dermal microvascular endothelium, normal | CRL-4045 | TIME-GFP |
| Skin | hTERT human foreskin keratinocyte, normal | CRL-4048 | Ker-CT |
| Skin | hTERT human dermal microvascular endothelium, normal | CRL-4049 | NFkB-TIME |
| Skin | hTERT dermal melanocyte, normal, adult | CRL-4059 | hTERT-immortalized Dermal Melanocyte |
| Skin | hTERT dermal microvascular endothelium, neonatal | CRL-4060 | hTERT Dermal Microvascular Endothelial Cell, Neonatal |
| Umbilical cord | hTERT human umbilical vascular endothelium, normal | CRL-4053 | HUVEC/TERT 2 |
| Uterus | hTERT human endometrium fibroblast, non-malignant myoma | CRL-4003 | T HESCs |
Applications
1. Long-term studies of biochemical and physiological aspects of cell growth; 2. In vitro model for differentiation and carcinogenesis; 3. Cancer research and studies of oncogenes; 4. Genetic engineering and modifications; 5. Tissue engineering, and transplantation; 6. Gene expression studies; 7. Cell-based drug screening, and drug toxicity testing; 8. Biological functions of the hTERT
For information about depositor, immortalization method, growth medium, karyotype, and references please consult the appropriate product detail page.
References
- Shay JW, Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 2005; 26: 867-874.
- Palanca-Wessels MC, Barrett MT, Galipeau PC, Rohrer KL, Reid BJ, Rabinovitch PS. Genetic analysis of long-term Barrett’s esophagus epithelial cultures exhibiting cytogenetic and ploidy abnormalities. Gastroenterology 1998; 114: 295-304.
- Rambhatla L, Chiu CP, Glickman RD, Rowe-Rendleman C. In vitro differentiation capacity of telomerase immortalized human RPE cells. Invest Ophthalmol Vis Sci 2002; 43: 1622-1630.
- Arbiser JL, Yeung R, Weiss SW, Arbiser ZK, Amin MB, Cohen C, Frank D, Mahajan S, Herron GS, Yang J, Onda H, Zhang HB, Bai X, Uhlmann E, Loehr A, Northrup H, Au P, Davis I, Fisher DE, Gutmann DH. The generation and characterization of a cell line derived from a sporadic renal angiomyolipoma: use of telomerase to obtain stable populations of cells from benign neoplasms. Am J Pathol 2001; 159: 483- 491.
- Lim SD, Stallcup W, Lefkove B, Govindarajan B, Au KS, Northrup H, Lang D, Fisher DE, Patel A, Amin MB, Arbiser JL. Expression of the neural stem cell markers NG2 and L1 in human angiomyolipoma: are angiomyolipomas neoplasms of stem cells? Mol Med 2007; 13: 160-165.
- Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V, Der CJ. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res 2007; 67: 2098-2106.
- Zabrenetzky V, et al. In: Annual meeting of the ASCB; 1997.
- Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 2004; 64: 9027-9034.
- Haga K, Ohno S, Yugawa T, Narisawa-Saito M, Fujita M, Sakamoto M, Galloway DA, Kiyono T. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT. Cancer Sci 2007; 98: 147-154.
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279: 349-352.
- Venetsanakos E, Mirza A, Fanton C, Romanov SR, Tlsty T, McMahon M. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp Cell Res 2002; 273: 21-33.
- Herbert BS, Wright WE, Shay JW. p16(INK4a) inactivation is not required to immortalize human mammary epithelial cells. Oncogene 2002; 21: 7897-7900.
- Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, von Werder A, Enders GH, Opitz OG, Rustgi AK. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res 2003; 1: 729-738.
- Thi MM, Urban-Maldonado M, Spray DC, Suadicani SO. Characterization of hTERT-immortalized osteoblast cell lines generated from wild-type and connexin43-null mouse calvaria. Am J Physiol Cell Physiol; 299: C994-C1006.
- Lee KM, Nguyen C, Ulrich AB, Pour PM, Ouellette MM. Immortalization with telomerase of the Nestin- positive cells of the human pancreas. Biochem Biophys Res Commun 2003; 301: 1038-1044.
- Li H, Zhou J, Miki J, Furusato B, Gu Y, Srivastava S, McLeod DG, Vogel JC, Rhim JS. Telomerase- immortalized non-malignant human prostate epithelial cells retain the properties of multipotent stem cells. Exp Cell Res 2008; 314: 92-102.
- Toouli CD, Huschtscha LI, Neumann AA, Noble JR, Colgin LM, Hukku B, Reddel RR. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene 2002; 21: 128-139.
- Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, Sarosi GA, Jr., Spechler SJ, Souza RF. Characterization of telomerase-immortalized, non-neoplastic, human Barrett’s cell line (BAR-T). Dis Esophagus 2007; 20: 256-264.
- Dalerba P, Guiducci C, Poliani PL, Cifola I, Parenza M, Frattini M, Gallino G, Carnevali I, Di Giulio I, Andreola S, Lombardo C, Rivoltini L, Schweighoffer T, Belli F, Colombo MP, Parmiani G, Castelli C. Reconstitution of human telomerase reverse transcriptase expression rescues colorectal carcinoma cells from in vitro senescence: evidence against immortality as a constitutive trait of tumor cells. Cancer Res 2005; 65: 2321-2329.
- Delgado O, Kaisani AA, Spinola M, Xie XJ, Batten KG, Minna JD, Wright WE, Shay JW. Multipotent capacity of immortalized human bronchial epithelial cells. PLoS One; 6: e22023.
- Vaughan MB, Ramirez RD, Andrews CM, Wright WE, Shay JW. H-ras expression in immortalized keratinocytes produces an invasive epithelium in cultured skin equivalents. PLoS One 2009; 4: e7908.
- Zhao X, Malhotra GK, Lele SM, Lele MS, West WW, Eudy JD, Band H, Band V. Telomerase-immortalized human mammary stem/progenitor cells with ability to self-renew and differentiate. Proc Natl Acad Sci U S A; 107: 14146-14151.
- Cao H, Chu Y, Zhu H, Sun J, Pu Y, Gao Z, Yang C, Peng S, Dou Z, Hua J. Characterization of immortalized mesenchymal stem cells derived from foetal porcine pancreas. Cell Prolif; 44: 19-32.
- Noble JR, Zhong ZH, Neumann AA, Melki JR, Clark SJ, Reddel RR. Alterations in the p16(INK4a) and p53 tumor suppressor genes of hTERT-immortalized human fibroblasts. Oncogene 2004; 23: 3116-3121.
- Waki K, Anno K, Ono T, Ide T, Chayama K, Tahara H. Establishment of functional telomerase immortalized human hepatocytes and a hepatic stellate cell line for telomere-targeting anticancer drug development. Cancer Sci; 101: 1678-1685.
- Kassem M, Abdallah BM, Yu Z, Ditzel N, Burns JS. The use of hTERT-immortalized cells in tissue engineering. Cytotechnology 2004; 45: 39-46.
- Loughery JE, Dunne PD, O’Neill KM, Meehan RR, McDaid JR, Walsh CP. DNMT1 deficiency triggers mismatch repair defects in human cells through depletion of repair protein levels in a process involving the DNA damage response. Hum Mol Genet; 20: 3241-3255.
- Gorbunova V, Seluanov A, Pereira-Smith OM. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J Biol Chem 2002; 277: 38540-38549.
All hTERT products are distributed under the terms and conditions of (i) the ATCC Material Transfer Agreement, and (ii) the Addendum to the MTA for Screening Applications ("Screening Addendum") if the customer is a for-profit.
The TERT-containing plasmid is not available to commercial and for-profit organizations or for work to be conducted under funding from a commercial organization unless a commercial license is obtained. For information please e-mail ATCC’s Office of IP, Licensing and Services at [email protected].