Chronic diseases, which are conditions that persist for a year or more and require ongoing medical care or limit daily activities, are the primary causes of death and disability in the United States and a leading contributor to healthcare costs.1-3 Among these, cancer poses a substantial challenge, requiring ongoing research and innovative approaches. Since 2016, ATCC has partnered with the National Cancer Institute (NCI) to support various programs and initiatives, such as the Human Cancer Models Initiative (HCMI), by providing well-characterized cancer cell lines and 3-D models, such as organoids, that facilitate translational cancer research and help identify and target novel therapies. HCMI tumor tissues are derived from 18 tissue sites and represent diverse genetic backgrounds, ages, sexes, treatment information, clinical tumor diagnosis, and clinical stages. HCMI research institutions deposit the models into ATCC, where they are authenticated, expanded, preserved, and made available for global distribution. ATCC distributes inventoried clinical samples while also managing over 22 million irreplaceable clinical samples at NCI’s biorepository in Frederick, Maryland, to advance ground-breaking cancer research. Learn more about these programs in our short videos: ATCC's Collaboration with HCMI and 3-D In Vitro Models & Organoids.
In 2021, reports showed chronic cardiovascular and respiratory diseases like ischemic heart disease, stroke, and chronic obstructive pulmonary disease (COPD) resulted in 13%, 10%, and 5%, respectively, of the world’s total deaths.4 In order to facilitate research in respiratory diseases, ATCC has developed in vitro cellular and organoid models for COPD and asthma. These models offer a wide variety of respiratory primary cell lines from normal and diseased patients, including lung fibroblasts, bronchial/tracheal, lobar, and small airway epithelial cells. ATCC has also enabled a physiologically relevant 3-D lung organoid model using primary human bronchial/tracheal epithelial cells [HBECs (ATCC® PCS-300-010™)] on an air-liquid interface (ALI) culture model, which is ideal for studies on microbial infection and pathogenesis, airway inflammation, wound healing, and toxicology.5,6 ATCC has conducted research into 2-D and 3-D cardiac organoid models using differentiated cardiomyocytes from human induced pluripotent stem cell-cardiomyocytes (hiPSC-CM) and ATCC Maturation Reagent (AMR™).7 These organoids may allow for better in vitro modeling of cardiopulmonary chronic disease for pathological, pharmacological, or therapeutic purposes.
Due to the increase in the elderly population in recent years, neurodegenerative diseases like Alzheimer's and Parkinson's are becoming increasingly prevalent, leading to a surge in research aimed at understanding the causes and mechanisms of each disease.8 For this purpose, ATCC has produced normal (ATCC® ACS-5004™) and Parkinson’s disease iPSC-derived neural progenitor cells (ATCC® ACS-5001™) that differentiate into mature neuronal cell types. Normal iPSC-derived neural progenitor cells are key in Alzheimer’s research.9 In addition, ATCC has partnered with the Michael J. Fox Foundation for Parkinson’s Research to provide vital cell lines, including macrophage LRRK2 and SNCA knockout isogenic cell line collections, to academic, pharmaceutical, and biotechnology organizations committed to speed a cure for Parkinson’s disease.
Metabolic syndrome is characterized by a group of risk factors, including hypertension, hyperglycemia, dyslipidemia, and central obesity, that collectively increase the risk of developing chronic diseases such as type 2 diabetes mellitus and cardiovascular disease.10 Disturbances in white and brown adipocyte metabolism may contribute to the causes of diabetes and obesity. ATCC possesses hTERT-immortalized brown (hTERT-hB; ATCC® CRL-4062™) and white (hTERT-hWA; ATCC® CRL-4063™) pre-adipocytes that have been developed and characterized in collaboration with Dr. Aaron Cypess at the National Institutes of Health for studying their roles in metabolic disease.11 Future research into the role of the human microbiome in the development of chronic diseases like diabetes, irritable bowel syndrome (IBS), and Crohn’s disease may be aided by reagents and biological materials from ATCC and the Human Microbiome Project, available through BEI Resources.
Microorganisms play a significant role in chronic diseases, with certain infectious agents like hepatitis B and C viruses, human papillomavirus (HPV), human immunodeficiency virus (HIV), and Mycobacterium tuberculosis leading to severe long-term health consequences, such as liver cancer and cirrhosis, uterine cancer, Kaposi sarcoma, and respiratory damage, respectively.3 ATCC, through the Biological and Emerging Infections Research Resources Program (BEI-RRP; BEI Resources) contract, provides a wide variety of well-characterized challenge materials and authenticated biological reagents, including specific pathogen strains, purified pathogen DNA and RNA, and antibodies to pathogen molecules. In addition, an extensive database of pathogen information maintained by BEI-RRP provides researchers with vital information about pathogens, such as the multidrug resistance profiles of M. tuberculosis strains.
In summary, ATCC plays a pivotal role in advancing research on chronic diseases by providing high-quality biological materials, innovative models, and services. Their comprehensive bioresource collection and partnerships with leading institutions bolster efforts in understanding and treating conditions such as cancer, pulmonary diseases, neurodegenerative disorders, metabolic diseases, and other infectious-induced chronic diseases. With these resources, researchers can explore novel therapies and gain deeper insights into the mechanisms of chronic illnesses, ultimately contributing to improved patient outcomes and healthcare solutions.
Did you know?
ATCC provides synthetic molecular standards for hepatitis B and C viruses, which can be used as positive controls in assays designed to detect and quantify these viruses in a person’s blood.12 These molecular standards allow for accurate detection and quantification of these viruses in the blood and may help health staff monitor the effectiveness of antiviral drugs used for the treatment of hepatitis B and C viruses.
Meet the author
Malcolm Barth, PhD
Technical Writer, ATCC
Dr. Barth is a technical writer for the Business Development Unit at ATCC Federal Solutions (AFS) within ATCC. He holds a PhD in Cancer Immunology from the Medical College of Pennsylvania. He holds master’s degrees in Medical Microbiology and Regulatory Science from the University of Manitoba and the University of Southern California, respectively. Dr. Barth has worked in science as a researcher; in human subject protection roles with Institutional Review Boards; as a chemistry, biology, and microbiology instructor in high schools and undergraduate institutions; and as a science writer and medical writer. As a technical writer at ATCC, his work has involved writing research proposals, biosketches, blog posts, and white papers and reviewing research proposals for scientific accuracy.
Explore our featured resources

Federal Solutions
Support for federal agencies through contracts focused on global health and infectious diseases, biodefense, chronic diseases, clinical study support, global logistics, and biorepository establishment/maintenance.
More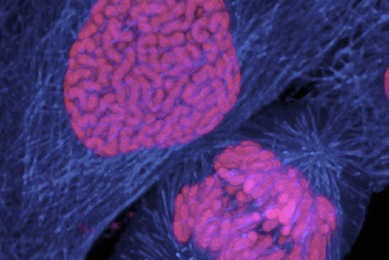
Cancer Research
Fighting cancer requires painstaking research and development. Scientists need materials and standards for drug screening, tumor mechanisms, cancer immunology, and cancer diagnostics. ATCC has research models such as organoids, conditionally reprogrammed cells, luciferase expressing reporter cell lines, isogenic CRISPR/Cas9 genome-edited cell lines, and epithelial-mesenchymal transition reporter cell lines.
More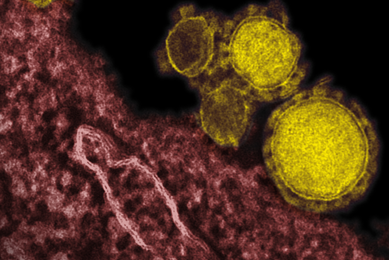
Infectious Disease Research
ATCC offers an extensive array of microorganisms and nucleic acids that promote research leading to the development of novel methods for detecting, minimizing, and treating infectious diseases.
MoreReferences
- Centers for Disease Control and Prevention. Leading Causes of Death. Webpage updated October 25, 2025. Accessed online: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- Centers for Disease Control and Prevention. Chronic Disease Indicators (CDI). Webpage updated August 5, 2024. Accessed online: https://www.cdc.gov/cdi/
- Institute of Medicine (US) Forum on Microbial Threats; Knobler SL, O'Connor S, Lemon SM, et al., editors. The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary. Washington (DC): National Academies Press (US); 2004. OVERVIEW. Available from: https://www.ncbi.nlm.nih.gov/books/NBK83680/
- World Health Organization. The top 10 causes of death. Accessed online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Bérubé K, et al. Human Primary Bronchial Lung cell constructs: the new respiratory models. Toxicology 278(3): 311-318, 2010. PubMed: 20403407
- Grady K, Tyo K. Elevate Your Toxicity Assays: New Models with Biological Relevance and Predictability. Presentation. Accessed April 7, 2025 at https://www.atcc.org/resources/webinars/2021-webinars/elevate-your-toxicity-assays
- Tsang KM, et al. Advanced 2D and 3D Cardiomyocyte-based Models for Use in Drug Discovery. Microphysiological Systems World Summit (2024): Abstract #622. Accessed online at: https://www.atcc.org/resources/posters/2024-posters/advanced-2d-and-3d-cardiomyocyte-based-models-for-use-in-drug-discovery
- Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech 10(5): 499-502, 2017. PubMed: 28468935
- Jung YW, et al. Human induced pluripotent stem cells and neurodegenerative disease: prospects for novel therapies. Curr Opin Neurol 25(2): 125-130, 2012. PubMed: 22357218
- Alberti KGMM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16): 1640-1645, 2009. PubMed: 19805654
- Cypess, A. The Development of a Standard In Vitro Model for Studying Metabolic Diseases. Presentation. Accessed April 10, 2025 https://www.atcc.org/resources/webinars/2024-webinars/the-development-of-standard-in-vitro-models-for-studying-metabolic-diseases
- Christina H, Mittar D. Development of Synthetic Molecular Standards for Hepatitis B and Hepatitis C Virus. Application Note. Accessed April 7, 2025 https://www.atcc.org/resources/application-notes/development-of-synthetic-molecular-standards-for-hepatitis-b-and-hepatitis-c-virus