Authors: John Foulke, MS; Luping Chen, BS; Brian Della Fera, BS; Hyeyoun Chang, PhD; Kevin Tyo, PhD; Zhizhan Gu, PhD; and Fang Tian, PhD
Chimeric antigen receptor (CAR)-T cells have displayed remarkable efficacy in treating malignant cancers, particularly liquid tumors. In this study, we present CAR-T Target Luciferase Reporter Cells that have high endogenous expression of CD19, CD20, and HER2, which makes them more physiologically relevant as in vitro tools to develop adoptive CAR-T cell therapies.
Introduction
Results with Material and Methods
CD19 CAR-T in vitro killing assay of Raji-Luc2 and WIL2-S-Luc2
HER2 CAR-T in vitro killing assay of BT-474-Luc2
CD20 CAR-T in vitro killing assay of Daudi-Luc2
CD20 CAR-T in vitro killing assay of Farage-Luc2
Conclusions
References
Abstract
Chimeric antigen receptor (CAR)-T cells have displayed remarkable efficacy in treating malignant cancers, particularly liquid tumors. CAR-T cells have proven to be a new type of “living” therapeutic that harnesses the patient’s immune system to recognize specific tumor-associated antigens and redirects the engineered T cells to more specifically target tumor cells.1 Considerable research efforts have been invested into developing new CAR structures to increase the scope of targeted cancer types and raise their antitumor efficacy.2 Evaluating the biofunction of CAR-T cells in vitro typically involves a series of labor-intensive co-culture experiments and immunoassays, where reproducibility remains a challenge during the validation of new CAR-T cells due to donor-to-donor variation and other possible factors.3 In this study, we present CAR-T Target Luciferase Reporter Cells that have high endogenous expression of CD19, CD20, and HER2, which makes them more physiologically relevant as in vitro tools to develop adoptive CAR-T cell therapies. These liquid and solid tumor cell lines exhibit sensitive and stable luciferase reporter expression that can be used to measure the potency and efficacy of a wide range of CAR structures engineered into T cells for autologous therapy.
Download a PDF of this application note
Download the Application NoteIntroduction
The Promise of Immuno-oncology
CAR-T cell-based therapeutics have emerged as a promising immunotherapy for treating specific leukemias, lymphomas, and myelomas. In this exciting approach to treating refractory cancers, T cells are isolated from a patient’s blood via apheresis. The cells are then incubated with the cytokine interleukin 2 and anti-CD3 antibodies to stimulate proliferation. Ex vivo silencing of genes involved in graft rejection is conducted to aid in improving T-cell stability after infusion. Once expanded, the appropriate CAR is introduced into the cells by retroviral transduction. These effector CAR-T cells are then infused back into the patient where they can exert their cytotoxic effects on tumor cells.1
The Bottleneck
Considerable research efforts have been invested into developing new CAR structures to increase the scope of targeted cancer types and raise their antitumor efficacy. One of the bottlenecks in the process of CAR-T development is evaluating the biofunction of CAR-T cells. This in vitro process typically involves a series of labor-intensive co-culture experiments and immunoassays, where reproducibility remains a challenge during the validation of new CAR-T cells due to donor-to-donor variation and other possible factors. In addition to reproducibility being an issue, the validation assays themselves can be problematic.
For example, the use of the radioactive 51Cr release assay has a major drawback; data can only be acquired at a single time point. Moreover, reagent half-life, protective measures, and waste disposal are critical factors because of the assay’s intrinsic radioactivity. Nonradioactive assays for CAR-T functional evaluation are available, although these require a time-consuming labeling step and suffer from intra- and inter-assay variability stemming from inconsistent dye uptake and spontaneous dye leakage over the course of the assay.
The Solution
A method of studying CAR-T effector function that eliminates the concerns of 51CR release and dye loading assays is the bioluminescence (BLI) reporter assay. In BLI reporter assays, target cells that constitutively express luciferase are co-cultured with the candidate effector cells and cytotoxicity is monitored via loss of BLI signal.4 In addition to their ease of use, the luciferase-expressing target cells in BLI assays can improve interexperimental reproducibility. Additionally, cells derived from non-human sources may not accurately capture the complexity of human physiology, calling into question the relevance of those studies.
ATCC’s CAR-T Target Luciferase Reporter Cells were derived from a variety of highly malignant liquid and solid cancer types, namely B cell lymphoma, Burkitt’s lymphoma, Non-Hodgkin’s B cell lymphoma, and ductal breast carcinoma. These novel target cells were generated from parental tumor cells that have high endogenous expression of target antigens such as CD19, CD20, and HER2 (Figure 1). Stable luciferase-expressing clones were engineered to display high signal-to-noise ratios, aiding in data interpretation.
To generate the CAR-T Target Luciferase Reporter Cells, antibiotic selection and single cell sorting were performed to isolate stable clones with high luciferase expression via the introduction of a Lenti-LUC2 luciferase reporter into the parental cell lines. The target antigen and luciferase were then verified to have expression stability by comparing the low-passage and the high-passage reporter cells. Once stable clones were selected and the expression of antigen and luciferase was verified, the reporter cell lines were characterized and authenticated using tried-and-true methods such as short tandem repeat (STR) profiling, mycoplasma detection, and cell growth rate and morphology assays.
The performance of the CAR-T Target Luciferase Reporter Cells was verified in T cell co-culture experiments. Commercially available CAR-T cells targeting CD19, CD20, and HER2 were employed in this study, with which empty vector-transduced T cells from the same donor were paired as controls. The cytotoxicity of the CAR-T cells against target tumor cells was measured using a luciferase assay, a commercially available potency assay, and a bright field and fluorescence live cell imaging assay. Our results demonstrate that the luciferase reporter system is a simple, robust, and highly sensitive means to measure biological processes in cancer and T cell ex vivo co-cultures. In summary, CAR-T Target Luciferase Reporter Cells from ATCC are well-characterized tools with high reproducibility for studying CAR-T biofunction and validating new CAR-T agents for cancer immunotherapy.
Figure 1: CAR-T Target Luciferase Reporter Cells. Schematic showing CAR-T target cells with expression of CD19-positive WIL2-S-Luc2 and Raji-Luc2, CD20-positive Daudi-Luc2 and Farage-Luc2, and HER2-positive BT-474-Luc2 being surrounded and attacked by CD19-, CD20-, and HER2-targeting CAR-T cells, respectively. Created with BioRender.com.
Results with Materials and Methods
Generation of CAR-T Target Luciferase Reporter Cells
To generate the CAR-T Target Luciferase Reporter Cells, we selected human tumor cell lines exhibiting high endogenous expression of clinically relevant CAR-T target antigens (CD19, CD20, HER2) on the cell surface. WIL2-S (ATCC CRL-8885), Raji (ATCC CCL-86), Daudi (ATCC CCL-213), Farage (ATCC CRL-2630), and BT-474 (ATCC HTB-20) were transduced with a lentiviral plasmid expressing luciferase under the control of an EF1A promoter. The genetically modified cells were grown under antibiotic selection, and single cell sorting was performed to isolate individual clones that were verified to stably express luciferase (Table 1).
Table 1: CAR-T Target Luciferase Reporter Cells
| Designation | ATCC® No. | Disease | Target |
|---|---|---|---|
| WIL2-S-Luc2 |
CRL-8885-LUC2™ | B Cell Lymphoma | CD19 |
| Raji-Luc2 | CCL-86-LUC2™ | Burkitt’s Lymphoma | CD19 |
| Daudi-Luc2 | CCL-213-LUC2™ | Burkitt’s Lymphoma | CD20 |
| Farage-Luc2 | CRL-2630-LUC2™ | Non-Hodgkin’s B Cell Lymphoma | CD20 |
| BT-474-Luc2 | HTB-20-LUC2™ | Breast Ductal Carcinoma | HER2 |
Performance of CAR-T Target Luciferase Reporter Cells
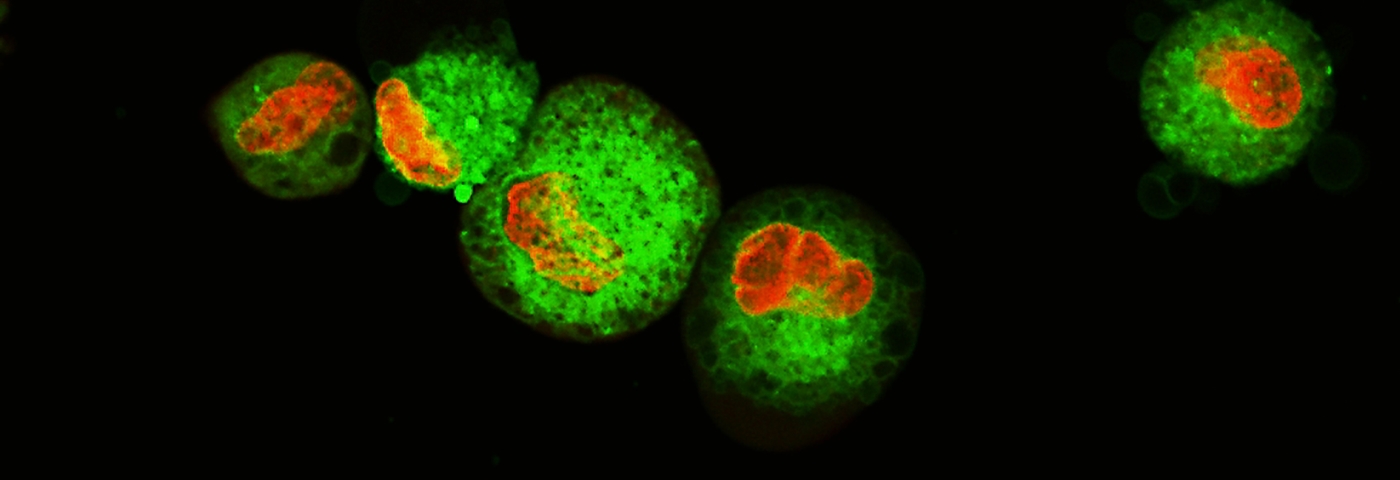
To demonstrate the performance of the CAR-T Target Luciferase Reporter Cells, we used cancer and T cell co-culture experiments. Commercially available CAR-T cells targeting CD19, CD20, and HER2 were employed in this study; empty vector-transduced T cells from the same donor were paired as controls. The cytotoxicity of the CAR-T cells against target tumor cells was measured using a luciferase assay, a commercially available potency assay, and a bright field and fluorescence live cell imaging assay. A luminescence assay was used to evaluate the functionality of the luciferase reporter cell lines in which the cells were co-cultured with antigen-specific CAR-T cells (ProMab) or mock CAR-T (ProMab) cells derived from the same donor as a control at various target to effector ratios (1:1, 2:1, 5:1, and 10:1). After co-culturing for 24 hours, the Bright-Glo™ Luciferase Assay System (Promega) was used and luminescence was measured on a plate reader. Analysis showed a clear dose-dependent decrease in luminescence, indicating the antigen-specific CAR-T killing potential was greater than the non-specific killing observed when co-culturing with mock CAR-T cells (Figures 2A, 2B, 3A, 4A, and 5A). Live cell imaging studies were conducted using the same method and were observed microscopically every 30 minutes to 1 hour using the Cytation 1 system (Agilent). The luciferase-expressing cells were either pre-stained with Vybrant™ (Thermo Fisher) DiO (Figure 2C and 2D,) or co-cultured in the presence of Incucyte® cytotox red dye (Sartorius)5 (Figures 4B and 5B). Incucyte cytotox red, which stains dead cells, shows a dose-dependent increase in fluorescence intensity when cells are co-cultured with antigen-specific CAR-T cells (Figures 4C, 4D, 5C, and 5D). BT-474-Luc2 when co-cultured with HER2 CAR-T cells or mock CAR-T cells from the same donor were measured in a real-time cell analysis assay using the xCelligence (Agilent)6 (Figure 3B) system and impedance was measured every 15 minutes. BT-474-Luc2 cells co-cultured with HER2 CAR-T cells show a greater decrease in cell impedance as the cells lift off the plate as compared to co-culturing with mock CAR-T cells or BT-474-Luc2 cells alone.
CAR-T Effector Cell Efficacy Using Raji-Luc2 and WIL2-S-Luc2
CAR-T Effector Cell Efficacy Using BT-474-Luc2 Cells
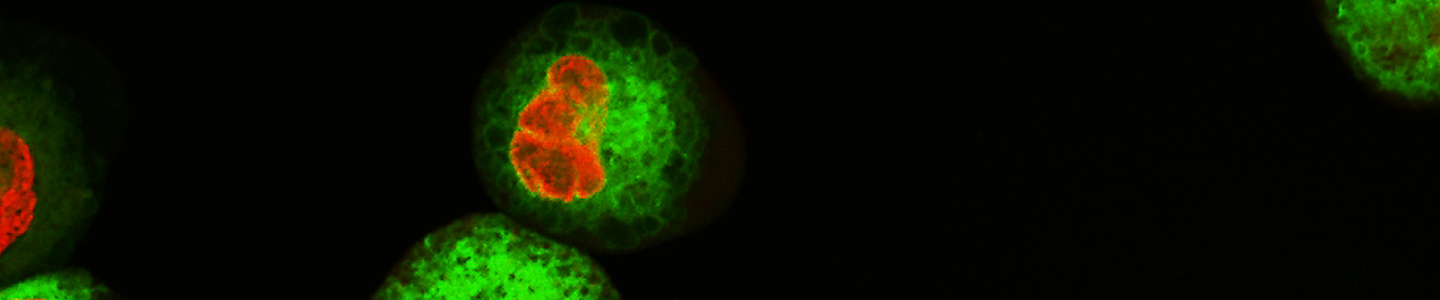
CAR-T Effector Cell Efficacy Using Daudi-Luc2 Cells
CAR-T Effector Cell Efficacy Using Farage-Luc2 Cells
Download a PDF of this application note
Download the Application NoteConclusions
The CAR-T Target Luciferase Reporter Cells described in this study provide an advantage in measuring target cell killing without the use of a radioactive 51Cr release assay or pre-labeling the cells for CAR-T functional evaluation.4 In addition, these reporter cell lines were characterized and authenticated using cell morphology, growth kinetics, and STR analysis.7 The expression stability of both the target antigen and luciferase was verified by comparing the low-passage and the high-passage reporter cells. Importantly, cytotoxicity in these luciferase-expressing cells could be measured by the loss of BLI signal in real time, and this loss was confirmed by live cell imaging and cytotoxic dye uptake assays. In summary, the well-characterized luciferase reporter cell lines enable convenient and consistent signal quantification, and they are easy-to-use tools for studying CAR-T biofunction and validating new CAR-T agents for cancer immunotherapy. These robust cell models are representative of the most predominant patient-derived carcinoma and lymphoma cancer lines used in oncology research. The CAR-T Target Luciferase Reporter Cells were selected from ATCC’s extensive catalog of established cancer cell lines that contain high endogenous expression of some of the most prevalent cancer antigens, which makes them more physiologically relevant as in vitro tools to develop adoptive CAR-T cell therapies.
Live-cell Imaging of CAR-T Cytotoxicity
References
- Kiesgen S, et al. Comparative analysis of assays to measure CAR T-cell-mediated cytotoxicity. Nat Protoc 16: 1331-1342, 2021. PubMed: 33589826
- Jackson HJ, et al. Driving CAR T-cells forward. Nat Rev Clin Oncol 13: 370-383, 2016. PubMed: 27000958
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 11: 69, 2021. PubMed: 33824268
- Riss T, et al. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells, in Assay Guidance Manual. (eds. S. Markossian et al.) (Bethesda (MD); 2004). PubMed: 31070879
- Meijer TWH, et al. Targeting glucose and glutamine metabolism combined with radiation therapy in non-small cell lung cancer. Lung Cancer 126: 32-40, 2018. PubMed: 30527190
- Lisby AN, et al. Evaluation of CAR-T cell cytotoxicity: Real-time impedance-based analysis. Methods Cell Biol 167: 81-98, 2022. PubMed: 35153000
- Capes-Davis A, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer 127: 1-8 2010. PubMed: 20143388