Authors: Aaron Briley, BS; Chengkang Zhang, PhD; and Brian Shapiro, PhD
Introduction
Materials and Methods
Results and Discussion
Conclusion
References
Abstract
This study will test the ability of primary human bronchial/tracheal epithelial cells (HBECs) to differentiate into mature airway tissue using an air-liquid culture interface (ALI) model.
Download a PDF of this application note
DownloadIntroduction
In the human body, the airway epithelium is characterized by a pseudostratified structure, ciliary motility, mucus secretion, and the formation of cellular junctions. Some of the major roles for HBECs include the absorption of extraneous molecules through the respiratory tract, the maintenance of epithelial homeostasis, remodeling, and the response to acute and chronic illnesses of the lung.1,2
Although most bronchial/tracheal airway functions are elucidated through in vivo/ex vivo studies, understanding the physiological and cellular processes that occur in the bronchial/tracheal epithelial cells of the human lung in normal and disease conditions is still very challenging due to the limitations of working with monolayer cell cultures in the laboratory. There is a growing need for physiologically relevant models to support future breakthrough research and drug discovery. With that in mind, ATCC investigated the properties of primary HBECs and validated them as an in vitro model to improve respiratory studies.
Materials and methods
Primary HBECs (ATCC PCS-300-010) were grown according to the ATCC product sheet recommendations. Falcon Permeable Supports (Corning) were placed into Costar 12 Well Clear Tissue Culture-Treated Multiple Well Plates (Corning) coated with Collagen Solution (STEMCELL Technologies) according to the manufacturer’s instructions, and incubated at 37°C overnight. Early passage HBECs were harvested, then resuspended in ATCC complete growth media, consisting of ATCC Airway Epithelial Cell Basal Medium (ATCC PCS-300-030) supplemented with Bronchial Epithelial Cell Growth Kit (ATCC PCS-300-040), at 180,000 cells/mL. The collagen-coated inserts were rinsed twice with Dulbecco’s Phosphate Buffered Saline (ATCC 30-2200), 1 mL of warm ATCC complete growth media was added to the basal chamber, and 500 μL of the cell suspension was seeded on the insert. The cells were incubated at 37°C until confluence was reached (2-4 days). Upon reaching confluence, the medium was removed from the inserts and from then on supplied only to the basal chamber (airlift). The PneumaCult-ALI protocol for Maintenance Phase (STEMCELL Technologies) was then followed for 21-28 days.3
Results and discussion
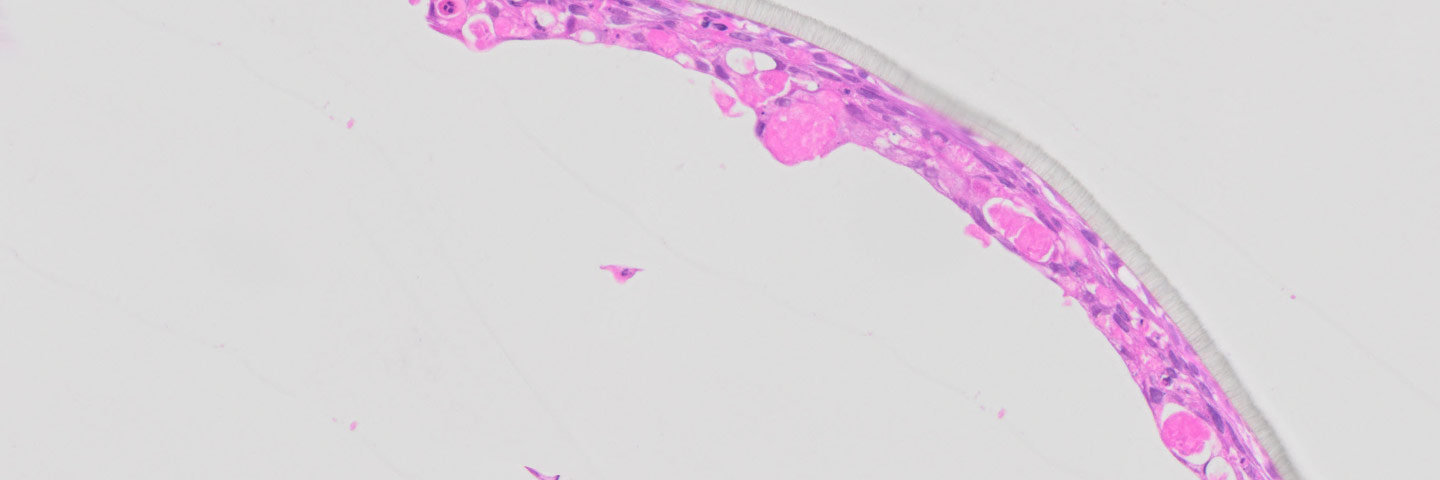
ATCC Primary HBECs cultured at the air-liquid interface for 28 days remained confluent and attached to the substrate (Figure 1), indicating their prolonged viability in an environment mimicking that of the respiratory epithelium. In addition, the ALI-cultured HBECs exhibited the characteristics of differentiated airway epithelia. For example, Hemotoxylin and Eosin (H&E)-staining indicated that the HBECs formed a pseudostratified, ciliated epithelium (Figure 2A; arrow indicates cilia). In addition, the cilia were observed to beat in a synchronous manner (data not shown). Moreover, some of the cells exhibited large, periodic acid-Schiff (PAS)/Alcian blue staining vesicles (Figure 2B; arrow indicates vesicle), suggesting that some of the cells differentiated into goblet cells.
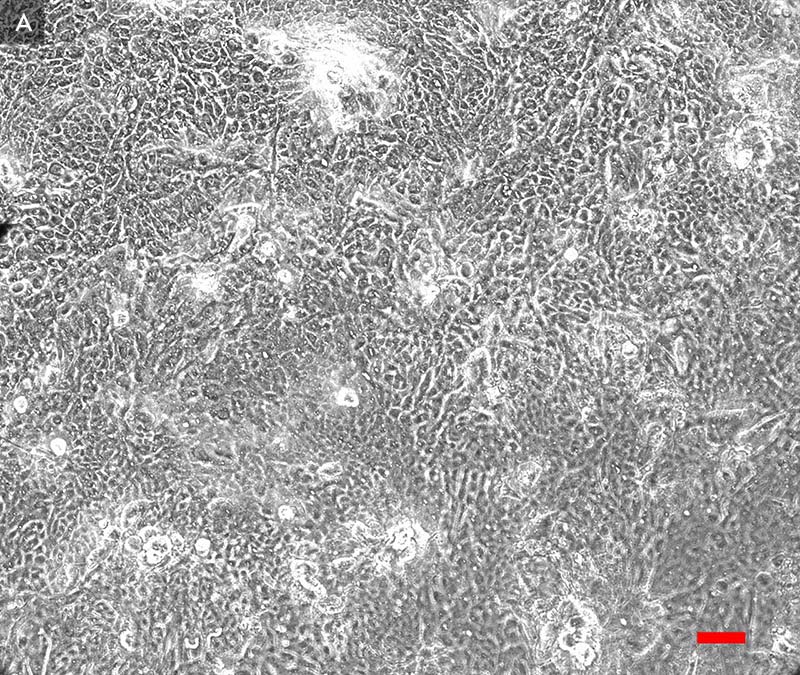
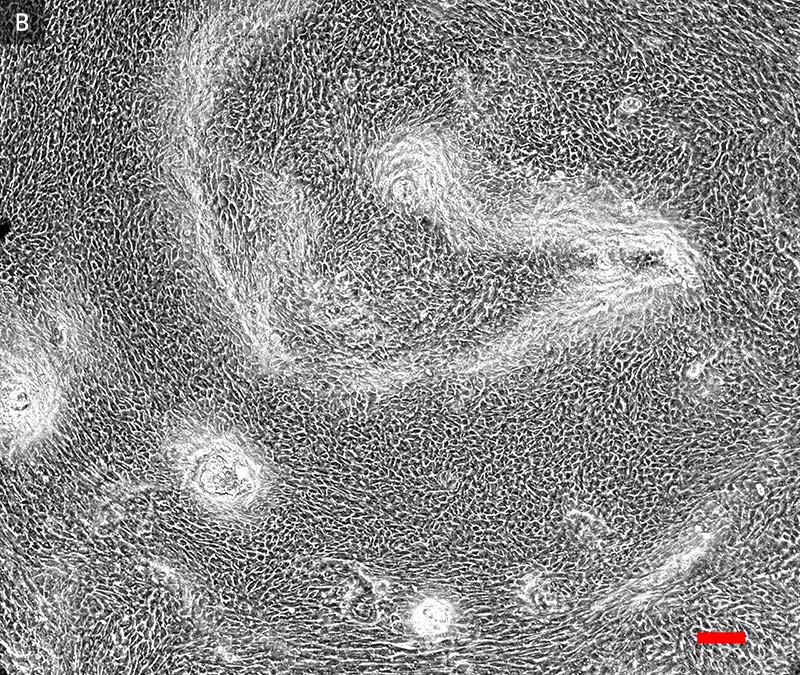
Figure 1. Phase contrast image (20x) of primary HBECs at A) 7 days and B) 28 days post airlift.
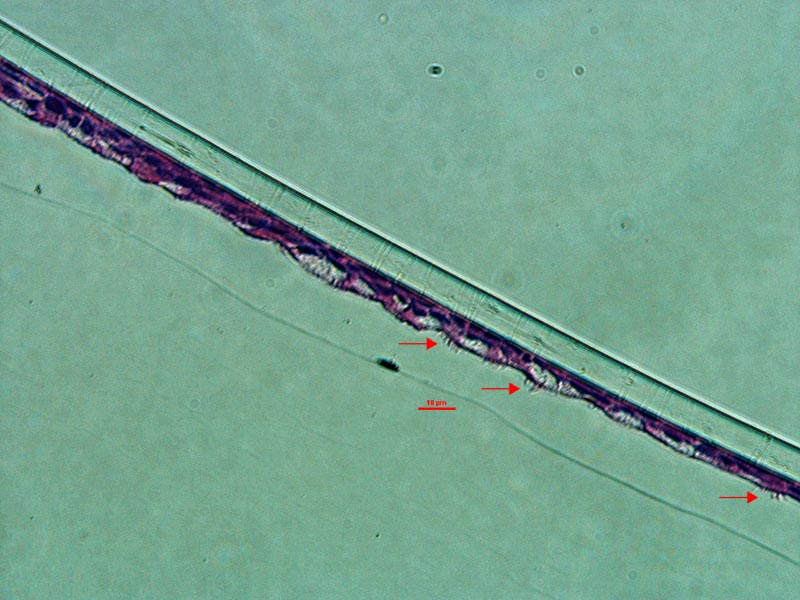
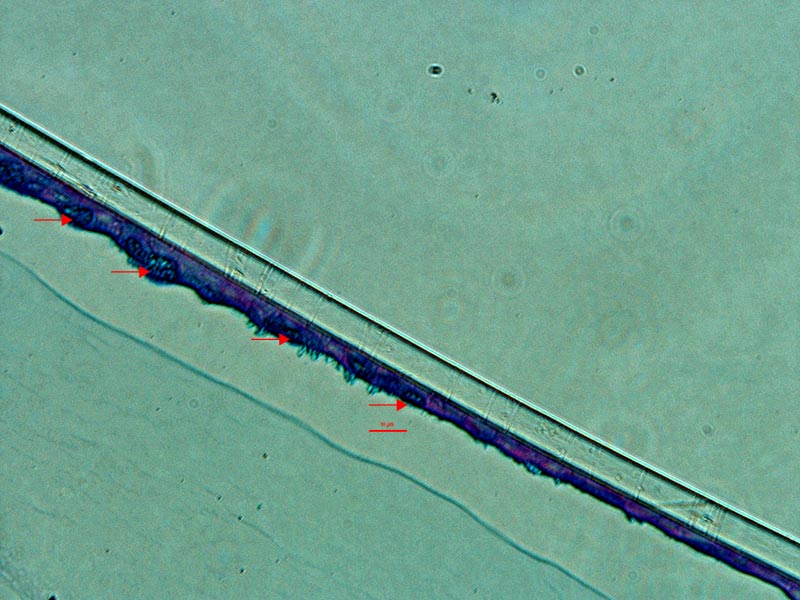
Figure 2. Micrograph (20x) of cross sections of primary HBECs at 28 days post airlift stained with A) H&E and B) PAS/Alcian blue.
Conclusion
ATCC Primary HBECs formed differentiated bronchial/tracheal epithelial structures at the air-liquid interface, resembling the airway epithelium. Thus, an excellent 3-D model has been established for the investigation of the bronchial/tracheal epithelium, disease modeling, toxicity studies, molecule absorption, and drug discovery, among other applications. It is a physiologically relevant system designed to improve respiratory studies and increase the success rate of translational research.
Download a PDF of this application note
DownloadReferences
- de Jong PM, et al. Ciliogenesis in human bronchial epithelial cells cultured at the air-liquid interface. Am J Respir Cell Mol Biol 10: 271-277, 1994.
- Berube K, et al. Human primary bronchial lung cell constructs: the new respiratory models. Toxicology 278: 311- 318, 2010.
- STEMCELL Technologies. PneumaCult-ALI Culture Medium for Air-Liquid Interface Culture of Human Bronchial Epithelial Cells. Product Information Sheet.