UPCI:SCC090
CRL-3239 ™
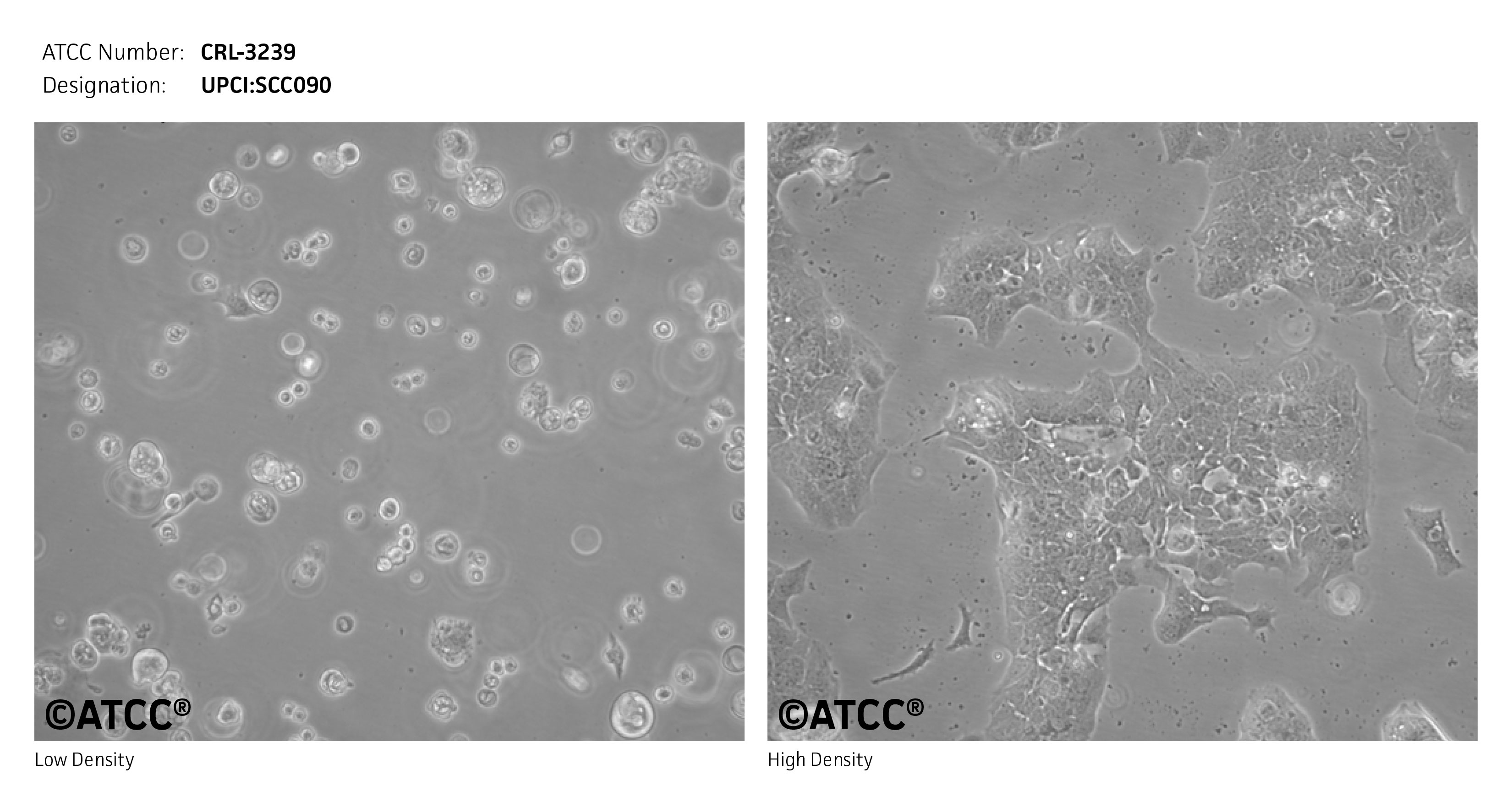
UPCI:SCC090 is a squamous cell line that was isolated in 1994 from a 46-year-old, white male patient with squamous cell carcinoma, grade 3, pathological stage: T2N0. These cells were isolated from the base of the tongue. They are positive for Human Papilloma Virus (HPV). They have no TP53 mutations as assayed by sequencing the 5-8 exons of TP53. UPCI:SCC090 showed no amplification of chromosomal band 11q13 using FISH.
The patient had a recurrence about 1 year later sited at the hypopharynx. It is ATCC No. CRL-3240 UPCI:SCC152. This cell line was deposited by Susanne M. Gollin, University of Pittsburgh, and can be used in studying the initiation, cancerization, prognosis, intervention and treatment of oral cancers.