
ATCC cultures have been specified and cited in national and international standards for many years. Examples include the standards promulgated by the Clinical and Laboratory Standards Institute (formerly NCCLS) for the healthcare community and by the United States Pharmacopeia (USP) for the pharmaceutical and biopharmaceutical industries. Cultures are used in performance testing of products, as positive and negative controls, as indicator organisms and as identification standards.
Though the use of microbiological standards is widely accepted, there is still some confusion as to specific laboratory guidelines, especially when determining the number of subcultures allowed beyond the reference strain. Discussions about passages have occurred on the Pharmaceutical Microbiological Mail List (PMFList).1-3 This Technical Document will attempt to clear up some of the confusion about passage and microbial culture maintenance and provide some definitions and recommendations.
Strain definitions
The confusion starts with the different names that are ascribed to reference strains. In various CLSI and USP publications these cultures are called control strains, standard cultures, reference strains, test strains, and quality control strains. These terms can generally be used interchangeably, though the preference seems to be reference strain or reference culture. Both the CLSI and USP agree that reference strains should come from a reliable source; both organizations cite ATCC. There is agreement that the reference strains from ATCC are subcultured to make “stock cultures,” which are subcultured weekly or monthly to make the “working cultures” used daily. Working cultures are often kept as slants, and it is these subcultures that raise the questions about passages from the original reference strain.
A subculture is a passage. The USP 36-NF 31 <51> Antimicrobial Effectiveness Testing, states: “For the purposes of the test, one passage is defined as the transfer of organisms from an established culture to fresh medium. All transfers are counted.”4
This definition was updated in the USP <1117> Microbiological Best Laboratory Practices to read: “One passage is defined as the transfer of organisms from a viable culture to fresh medium with growth of the microorganisms. Any form of subculturing is considered to be a transfer/passage.”5
This updated definition is preferable. The earlier definition left questions about the meaning of “an established culture.” There were several questions raised on the PMFList as to whether the frozen or freeze-dried vial from ATCC was an established culture. To some, the phrase “established culture” implied a growing culture. It is clear, however, that these frozen or freeze-dried vials of reference strains from ATCC are indeed “viable” cultures. A passage involves growing the microorganism with fresh medium, either on solid agar or in broth. Resuscitating frozen or freeze-dried cultures by thawing or rehydrating is not by itself considered a passage. Thus subculturing the reference strain from ATCC’s frozen or freeze-dried vial to the stock culture is the first passage. Subculturing stock cultures to working cultures is the second passage from the original reference strain. Any subsequent subculture is another cumulative passage.
Authenticity of cultures
Another term used by commercial sources other than ATCC is “ATCC derived.” A culture is derived from the ATCC reference strain by subculturing in other words, one or more passages. CLSI recognizes reliable commercial sources for reference strains. However, you must take into account that these cultures are at least one or two passages from the ATCC reference strains.
Maintaining cultures
The USP recommends a seed lot system to maintain reference strains in laboratories. In this system, the ATCC reference strain is subcultured to several replicates at one time, all of which are within one passage. These replicates of the stock culture are the seed vials for the laboratory. The seed stock is subcultured—the second passage—to make replicates of working cultures. This is the same system used at ATCC. (Figure 1).
But how many passages are recommended or acceptable in the laboratory? There is agreement that the number of passages should be minimized to reduce the possibility of phenotypic variations, genetic drift, and contamination as much as possible, but standards organizations differ as to how many passages are acceptable.
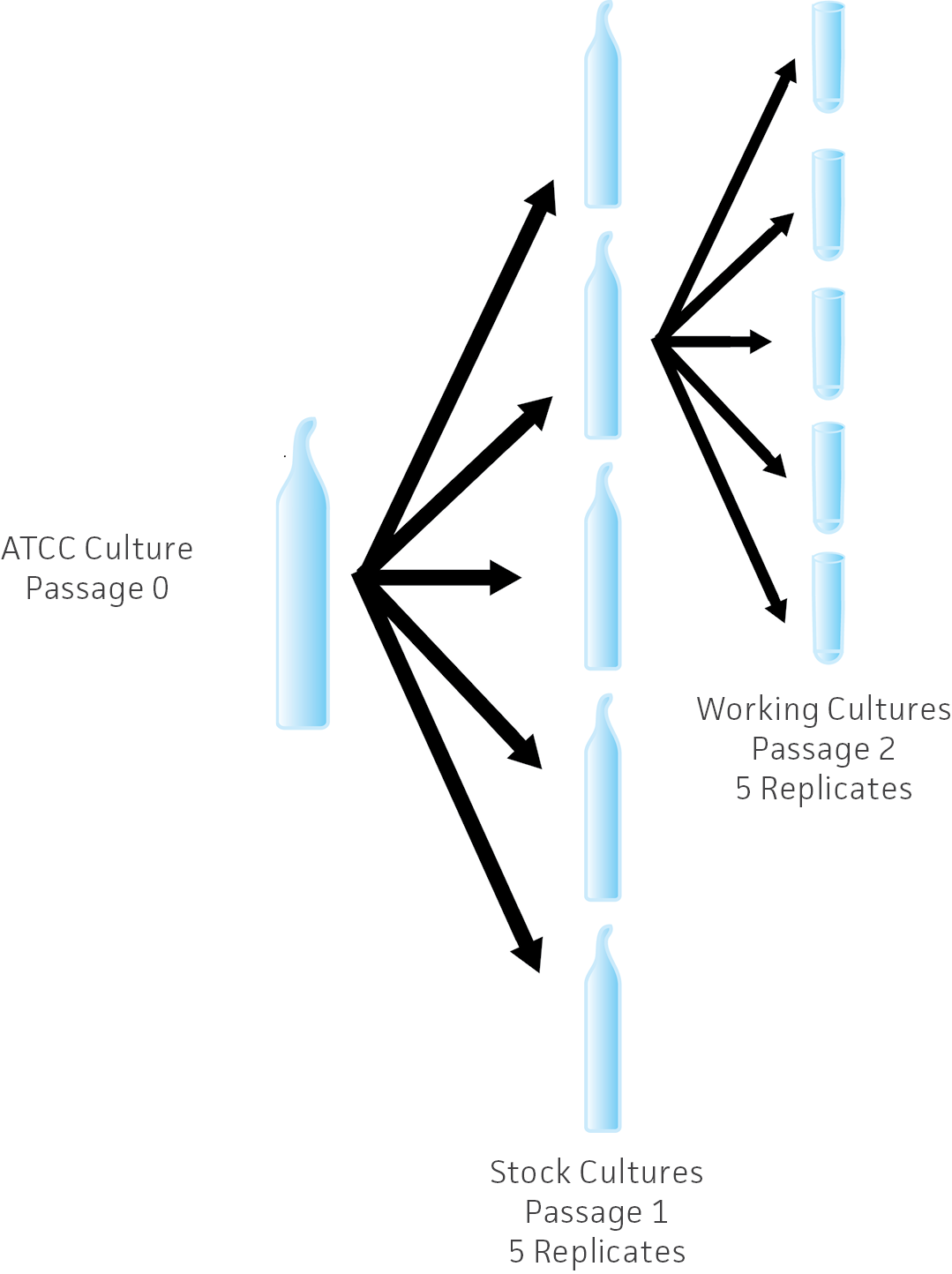
Figure 1. Seed lot system
Over the years, the recommendations from CLSI have varied. The least specific recommendations call for subculturing stock cultures weekly with new working cultures subcultured monthly, with no maximum number of passages noted. Another CLSI standard recommends up to three subcultures of the stock cultures and up to three subcultures of the working cultures.6 This would add up to as may as seven passages from the original ATCC reference culture (one to make the first stock culture, plus three subcultures and three additional subcultures).
USP standards have been more specific. USP General Chapter: <1117> Microbiological Best Laboratory Practices states “The number of transfers of working control cultures should be tracked to prevent excessive subculturing that increases the risk of phenotypic alteration or mutation. The number of transfers allowable for specific compendial tests may be specified in that test.”5 USP clearly states that the working cultures used for testing should not be more than five passages from the ATCC reference culture. The USP 36-NF 31 <51> states: “The viable microorganisms used in the test must not be more than five passages removed from the original ATCC culture.”4
The USP 36-NF 31 General notices:8 Terms and Definitions also contains the following definition: “Microbial Strains – A microbial strain cited and identified by its ATCC catalog number shall be used directly or, if subcultured, shall be used not more than five passages removed from the original strain.”7
The recommendation of five passages or less from the ATCC reference culture has been broadly accepted in the healthcare community and the pharmaceutical and biopharmaceutical industries. ATCC agrees with this recommendation.
Utilizing cold storage
Storage temperature of stock and working cultures can affect growth characteristics and viability. The CLSI recommendations include storage at −50°C to −70°C for one year or below −70°C indefinitely,6 or −20°C or below (preferably below −70°C) for “prolonged” storage.8,9,12 Storage of slants is recommended at 2°C to 8°C for up to four weeks.8,9,11,12 The USP 36-NF 31 <51> recommends storage in liquid nitrogen or a mechanical freezer below −50°C.
For long-term storage of frozen cultures, ATCC recommends the vapor phase of liquid nitrogen or a mechanical freezer at −80°C. Immersion in liquid nitrogen is not recommended. Frozen cultures containing a final concentration of 50% glycerol as a cryoprotectant may be kept at −20°C for short-term storage (less than one month). Do not store frozen cultures in a freezer with a defrost cycle; this will expose the cultures to higher temperatures. Freeze-dried cultures should be stored at 2°C to 8°C. Slants can be kept at 2°C to 8°C for up to a week.
Please note that special considerations for the maintenance and storage of certain QC strains should be reviewed before handling the strain. Some QC strains, such as those with plasmid-mediated antimicrobial resistance, have been shown to spontaneously loose the plasmid when stored at temperatures above −60°C or if subcultured repeatedly.8,9,11,12 For additional information, please refer to a current version of the appropriate CLSI standard referenced here.
Conclusion: ATCC recommendations
- ATCC reference strains should be subcultured to replicate stock cultures in the laboratory. Stock cultures can be subcultured for working cultures weekly, typically kept as slants. A seed lot system is recommended.
- A passage is defined as a subculture involving growth of the viable microorganism with fresh medium. Thawing or rehydrating ATCC reference cultures is not a passage.
- Microorganisms for standard protocols should be used within five passages of the ATCC reference culture.
- Frozen cultures should be stored in the vapor phase of liquid nitrogen or in a mechanical freezer at −80°C or below. Freeze-dried cultures should be stored at 2°C to 8°C. Slants may be stored at 2°C to 8°C for up to a week.
Download a PDF of this technical document
Download NowReferences
- Friedel B. Culture passage guidelines. In: PMFList Archives [Internet]. [The Pharmaceutical Microbiology Mail List] 2003 Jan 2.
- Kramer K. Seed stock culture. In: PMFList Archives [Internet]. [The Pharmaceutical Microbiology Mail List] 2003 June 10.
- Anger C. Seed stock culture. In: PMFList Archives [Internet]. [The Pharmaceutical Microbiology Mail List] 2003 June 11.
- U.S. Pharmacopeia. Antimicrobial Effectiveness Testing. In: U.S. Pharmacopeia 36-NF 31, 2013 <51>. Rockville, MD: U.S. Pharmacopeia; 2013.
- U.S. Pharmacopeia. Microbiological Best Laboratory Practices, <1117>. USP 36-NF 31, 2013.
- Clinical and Laboratory Standards Institute (CLSI). Quality Control for Commercially Prepared Microbiological Culture Media. Approved Standard-Third Edition. CLSI document M22-A3. Wayne, PA Clinical and Laboratory Standards Institute; 2004.
- U.S. Pharmacopeia. General Notices. In: USP 36-NF 31. Rockville, MD: U.S. Pharmacopeia; 2013.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th ed. CLSI standard M02. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. 5th ed. CLSI standard VET01. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 4th ed. CLSI standard M27. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2021.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. CLSI standard M07. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
Reprinted from ATCC Connection 23(2): 6-7, 2003 and subsequently updated.