Primary and immortalized keratinocyte cultures are essential models for investigating the normal and pathological biology of the epidermis. In this study, we confirmed that an immortalized keratinocyte cell line, Ker-CT, is able to differentiate into organotypic skin equivalents in a 3-D culture model.
Introduction
Human keratinocytes are instrumental for the study of skin biology and the pathogenesis of skin-related disease.1 The limited lifespan of primary keratinocyte cultures greatly limit their potential for advanced mechanism studies. The Ker-CT cell line was developed by the introduction of retroviruses expressing human telomerase (hTERT) and mouse cyclin-dependent kinase 4 genes into human neonatal foreskin keratinocytes.2 Ker-CT cells express basal epidermal stem cell markers such as tumor protein 63 and keratin 5. These cells are also positive for telomerase expression, fail to senesce and continue proliferation after more than 250 population doublings, and up-regulate the expression of differentiation markers in response to increased calcium concentration in conventional 2-D culture.2 Based on these positive cell attributes we examined whether the Ker-CT cells could could recapitulate the epidermal organotypic structure when grown in a 3-D culture model system.
Download a PDF copy of this application note
Download NowMaterials and methods
A matrix containing 84% Eagle’s Minimum Essential Medium (EMEM; Lonza), 9.28% Fetal Bovine Serum (FBS; ATCC 30-2020), 1.48 mML-glutamine (ATCC 30-2214), 0.28% sodium bicarbonate, 0.84 mg/mL Rat Tail Collagen (BD Biosciences), and 4.4 × 104 cells/mL Primary Human Neonatal Dermal Fibroblasts (ATCC PCS-201-010) was cast into 3.0 µm transwell inserts (Corning) in deep 12-well plates (Corning). After culturing for 7 days at 37°C in a 5% CO2 incubator, the collagen plug contracts. Medium in the transwell was then removed from the apical chamber. Primary Human Neonatal Dermal Keratinocytes (ATCC PCS-200-010) or Ker-CT (ATCC CRL-4048) were seeded at 2 × 105 cells/well into a cloning ring that was placed on top of the matrix and incubated under the same conditions for 2 hours. Epidermalization medium (EPM).1 consisting of 73% Dulbecco’s Modified Eagle’s Medium (DMEM; ATCC 30-2002), 24% Ham’s F12 Medium, 4 mM L-glutamine, 0.15 µM hydrocortisone, 1X ITES Media Supplement (Lonza), 0.01 mM o-phosphorylethanolamine, 0.18 mM adenine, 0.004 µM progesterone, 0.02 nM triiodothyronine, and 0.1% FBS, was added to the apical and basal chambers of the transwell. After two days of incubation at 37°C, the media in the basal chamber was replaced with EPM 2, comprised of 48% DMEM, 48% Ham’s F12, 4 mM L-glutamine, 0.15 µM hydrocortisone, 1X ITES, 0.01 mM O-phosphorylethanolamine, 0.18 mM adenine, 0.02 nM triiodothyronine, and 2% FBS, while the media in the apical chamber was removed to create an air-liquid interface (ALI).3 The cells were allowed to differentiate for 11-21 days post airlift at which time they were fixed with 4% paraformaldehyde, cross sectioned, and either hematoxylin and eosin (H&E) stained or stained for specific markers using primary antibodies directed against keratin 14 (KRT14; Thermo Fisher Scientific) and filiggrin (Thermo Fisher Scientific). Secondary antibodies used for marker tests were goat anti-mouse 584 (Thermo Fisher Scientific) or goat anti-rabbit 488 (Thermo Fisher Scientific). See Appendix 1 for a flow diagram of the method.
Results and discussion
To evaluate the differentiation capability of Ker-CT cells in a 3-D organotypic skin equivalent model after consecutive sub-cultivations, we tested the Ker-CT cells at early passage (passage 6, 10 PDL post thaw) and late passage (passage 15, 36 PDL post thaw) after recovery from cryopreservation. Primary human foreskin keratinocytes at passage 2 after recovery from cryopreservation were used as positive controls for skin equivalent differentiation. As shown by H&E stain, primary keratinocytes developed into characteristic stratified epidermal architecture composed of proliferating basal and differentiated suprabasal keratinocytes, as well as an evident superficial stratum corneum after 11 days in ALI culture (Figure 1).
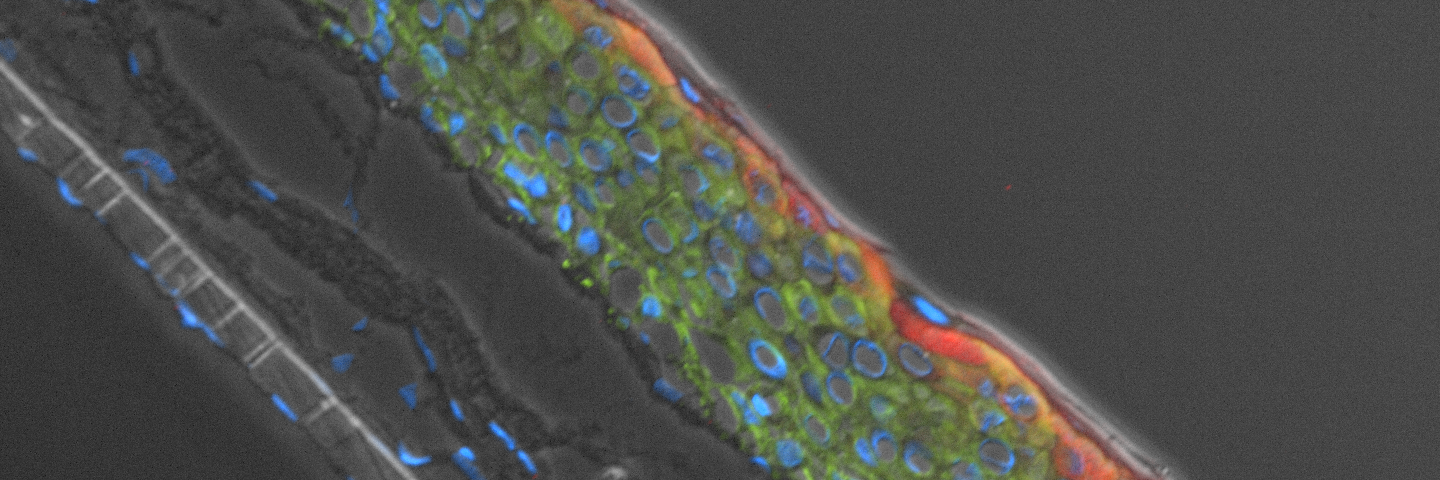
Ker-CT cells also developed into similar stratified epidermal architecture, although the stratum corneum was thinner than what was observed in primary keratinocytes. No apparent difference was seen between the early and late passages of Ker-CT cells (Figure 1). Further, after 21 days of ALI culture, Ker-CT cells at both early and late passages differentiated into characteristic epidermal structures that are reminiscent of primary keratinocytes (Figure 2). To verify the full differentiation of the cells, frozen cross sections were stained with antibodies directed against filaggrin, which stains for well-differentiated keratinocytes, and KRT14, which stains all epidermal cells. Filaggrin in both primary keratinocytes and Ker-CT displayed increased positivity in cells that are closer to the surface and nearing terminal differentiation. Both primary keratinocytes and Ker-CT cells displayed KRT14 immunoreactivity throughout the basal and suprabasal epidermal structure (Figures 3 and 4).
These data demonstrate that the immortalized Ker-CT cells retain their differentiation capability after an extended in vitro culture period beyond the capacity of normal primary keratinocyte culture. Thus Ker-CT cells have significant value for a wide variety of investigations of human epidermal biology in a tissue physiology-relevant context.
Conclusion
ATCC Ker-CT cells formed 3-D epidermal structures, rendering them an excellent and reproducible in vitro whole-cell model for the examination of mechanisms regulating keratinocyte biology in the processes of wound healing, dermal remodeling, cancer research, and drug toxicology evaluation in a tissue physiology-relevant context.
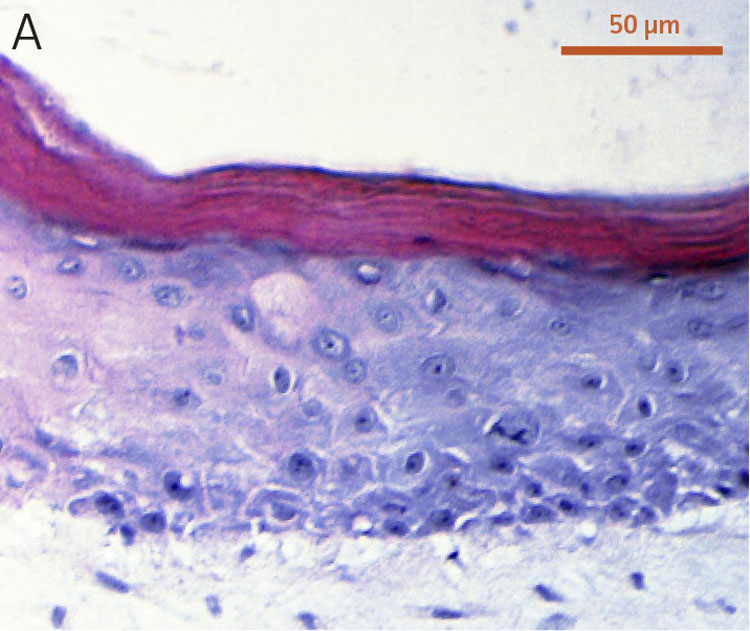
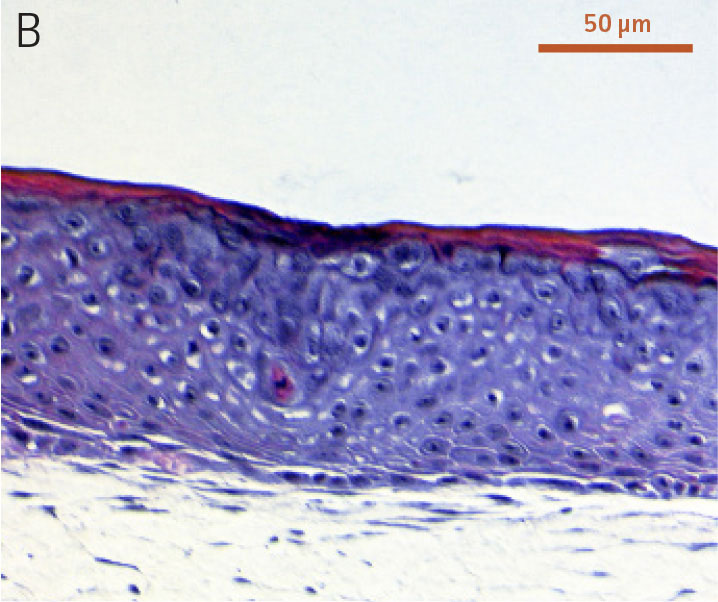
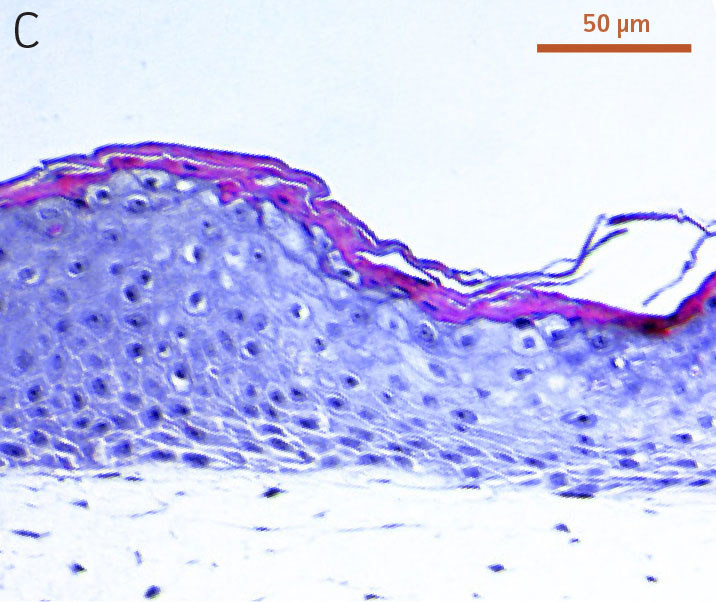
Figure 1. Micrograph of H&E-stained cross sections of A) primary foreskin keratinocytes at passage 2, B) Ker-CT at passage 6, and C) Ker-CT at passage 15 on top of a collagen gel embedded with primary foreskin dermal fibroblasts, after 11 days post ALI culture.
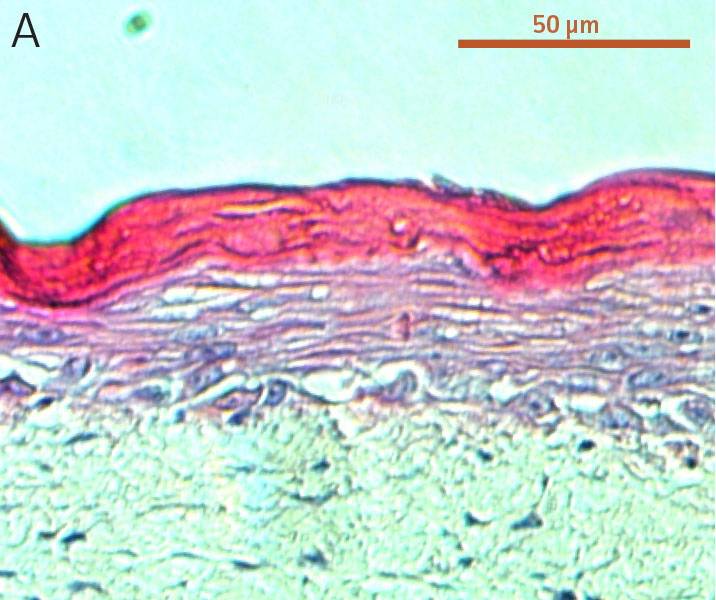
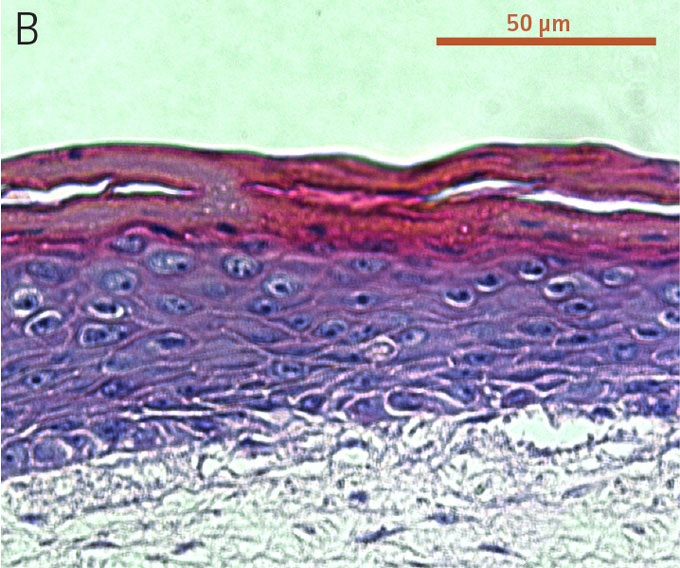
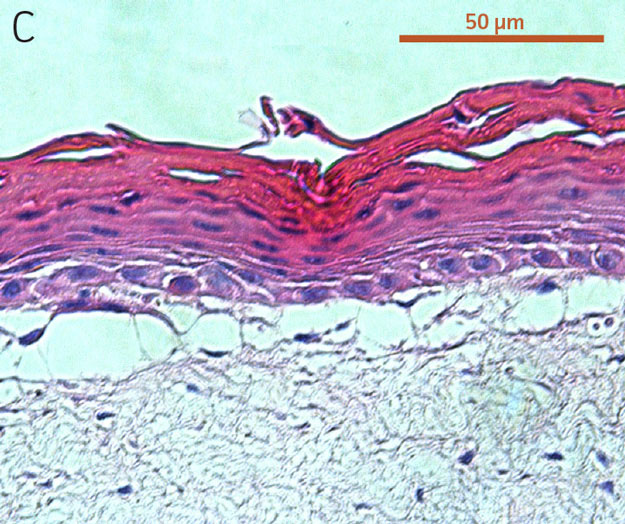
Figure 2. Micrograph of H&E-stained cross sections of A) primary foreskin keratinocytes at passage 2, B) Ker-CT at passage 6, and C) Ker-CT
at passage 15 on top of a collagen gel embedded with primary foreskin dermal fibroblasts, after 21 days post ALI culture.
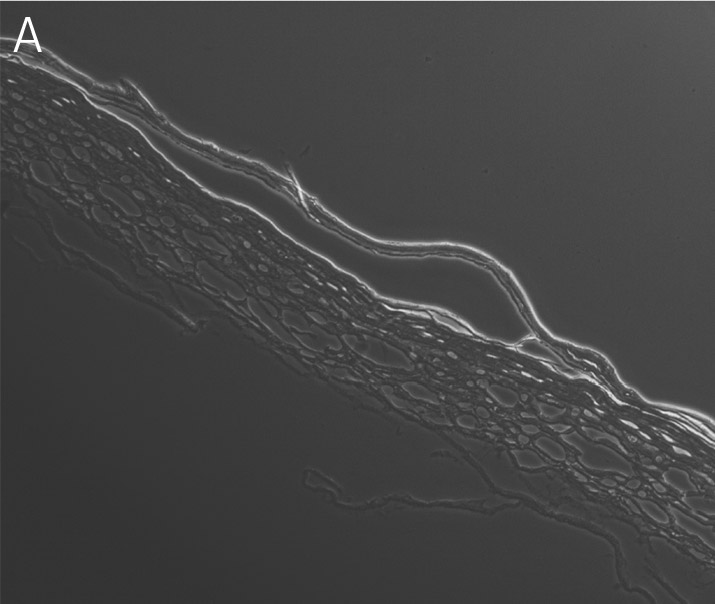

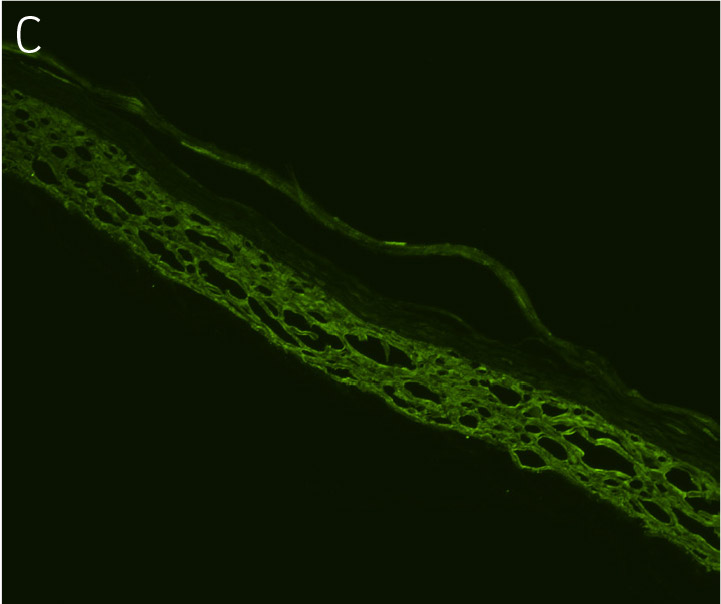
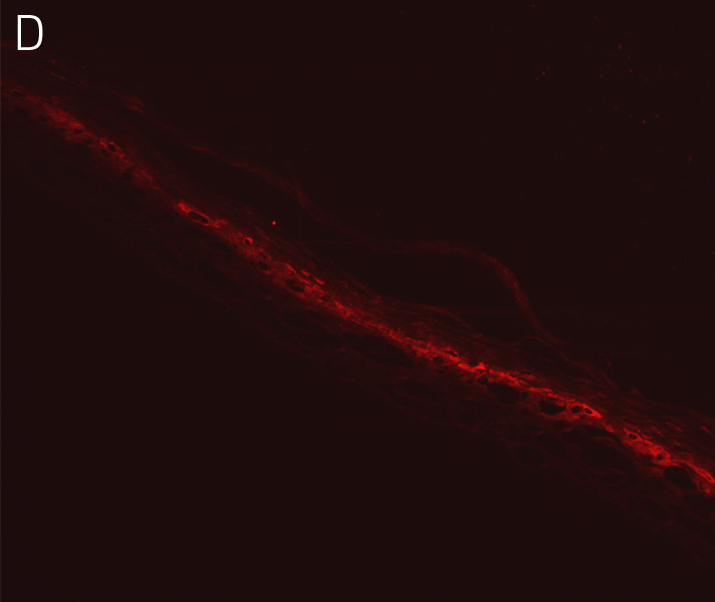
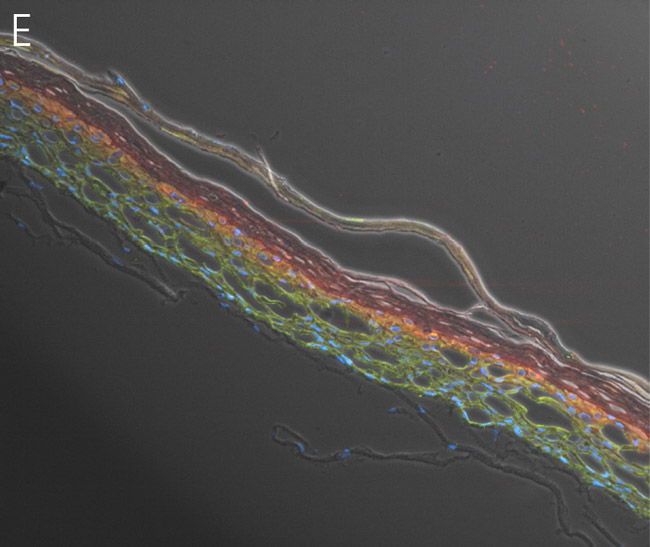
Figure 3. Primary keratinocytes cultured at ALI display similar architecture to skin in vivo. Primary keratinocytes were cultured on a collagen raft embedded with primary dermal fibroblasts at ALI for 11 days post airlift. Panel A) is a phase contrast micrograph at 10x magnification. Panels B-E show keratinocytes stained with B) DAPI or antibodies directed against C) KRT14, D) filaggrin, and E) overlay of all four channels.
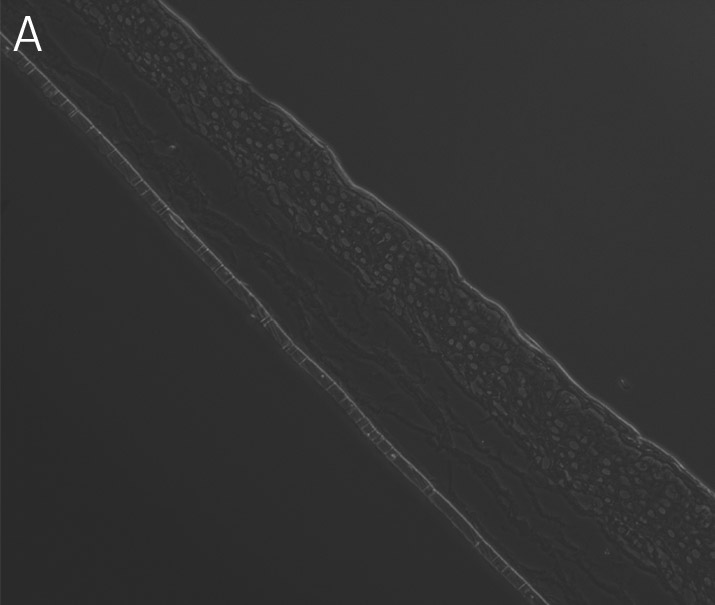
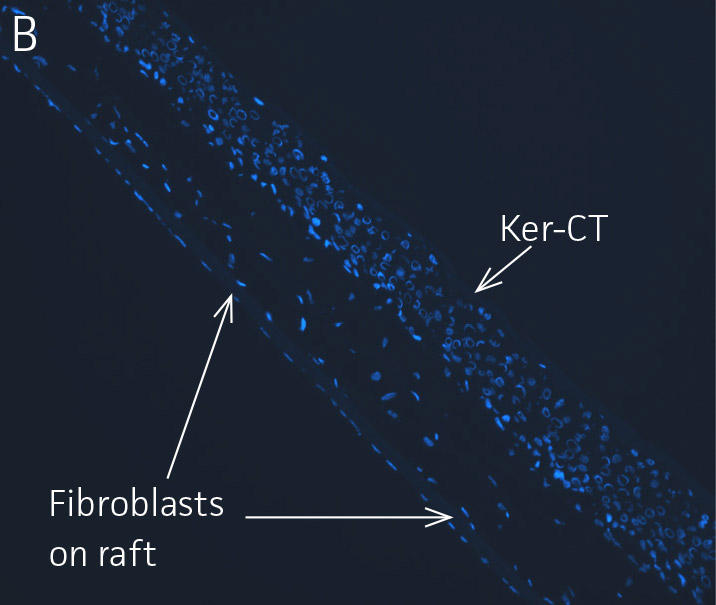
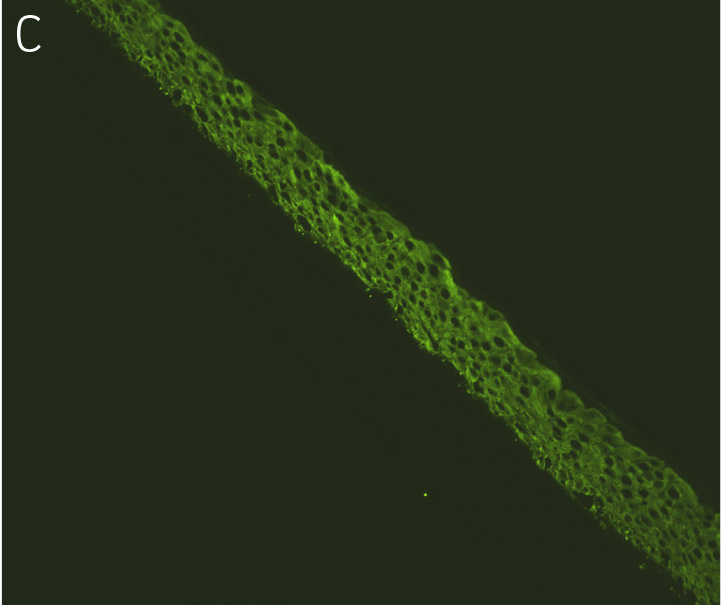
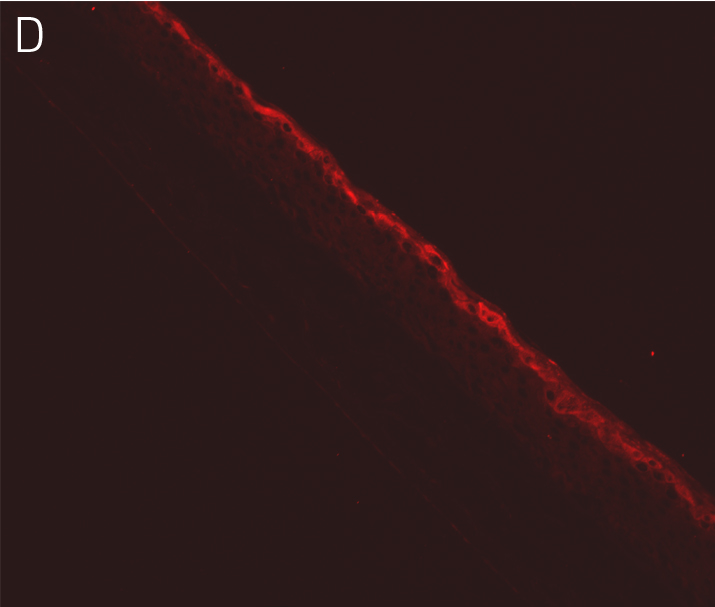
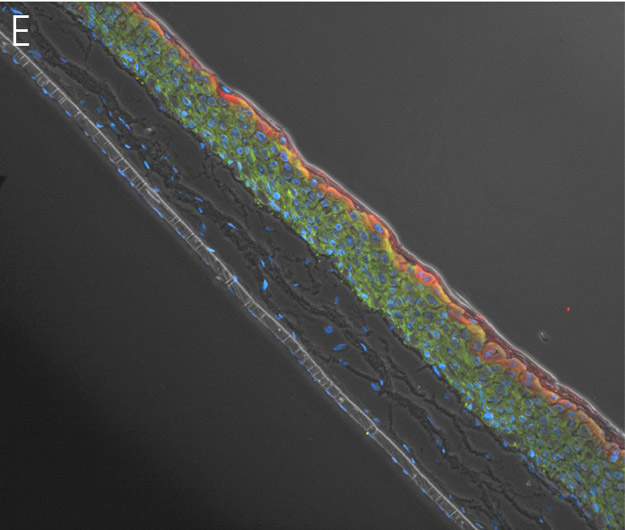
Figure 4. Ker-CT cultured at ALI display similar architecture to skin in vivo. Primary Ker-CT were cultured on a collagen raft embedded with primary dermal fibroblasts at ALI for 11 days post airlift. Panel A) is a phase contrast micrograph at 10x magnification. Panels B-E show Ker-CT stained with B) DAPI or antibodies directed against C) KRT14, D) filaggrin, and E) overlay of all four channels. Arrows indicate nuclei of Ker-CT and dermal fibroblasts.
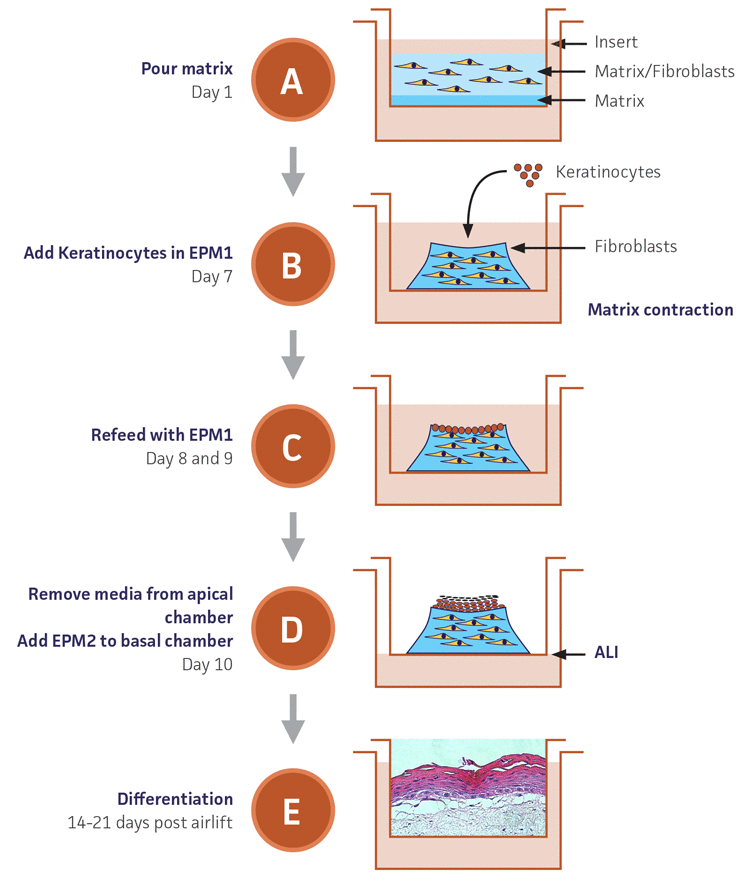
Appendix 1. Overview of co-culture of primary keratinocytes or Ker-CT grown on collagen raft embedded with primary dermal fibroblasts.
A) The raft co-culture is initiated by casting primary dermal fibroblasts in collagen on a collagen-coated insert in the well of a 12 well plate. The raft is submerged in a growth media for 7 days to promote the contraction of collagen for the formation of the raft structure. During this 7 day growth period, the fibroblasts grow into a mound-like cellular matrix.
B) On Day 7 the primary keratinocytes or the Ker-CT cells are seeded on top of the fibroblast mound and are allowed to attach.
C) EPM1, a keratinocyte proliferation growth medium, is added to the co-culture once a day for 2 days to assist the keratinocyte growth.
D) During the following 5-7 days the media is removed to allow an air-liquid interface, and EPM2, a keratinocyte maintenance medium, is added to the bottom of the well to assist in the continued growth and support of the keratinocytes.
E) The keratinocytes undergo differentiation for 14-21 days after day 11 of this treatment protocol.
Download a PDF copy of this application note
Download NowExplore our resources
References
- Gangatirkar P, et al. Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protoc 2(1):178-86, 2007. PubMed: 17401352
- Ramirez RD, et al. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of CDK4 in normal human epithelial cells. Oncogene 22(3):433-44, 2003. PubMed: 12545164
- Kalabis J, et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc 7(2):235-46, 2012. PubMed: 22240585