This study will determine if 3T3-L1 adipocyte differentiation is dependent on contact inhibition and will test the OP9 cell line as a possible substitute for 3T3-L1 cells in adipocyte assays.
Introduction
The mouse embryonic fibroblast cell line 3T3-L1 is a favored model for metabolism and obesity research because the cells can be chemically induced to differentiate into adipocytes.1 However, recent reports suggest that 3T3-L1 differentiation into adipocytes depends on the maintenance of contact inhibition and that 3T3-L1 cells can lose contact inhibition after several weeks if they are densely cultured.2 Here, we will test the claim that 3T3-L1 adipogenesis is contingent on the maintenance of contact inhibition and test the mouse bone marrow fibroblast cell line OP9, which does not lose contact inhibition despite being maintained densely in culture and may prove a suitable alternative to the 3T3-L1 for applications that require adipocytes.
Download a PDF of this application note
Download NowMaterials and methods
The 3T3-L1 cells (ATCC CL-173) were maintained at confluence in DMEM (ATCC 30-2002) supplemented with 10% Bovine Calf Serum (ATCC 30-2030) for three weeks to allow for loss of contact inhibition to occur. OP9 cells (ATCC CRL-2749) were maintained at confluence for 3 weeks in ALPHAμMEM without ribonucleosides and deoxyribonucleosides (Cellgro 15-012-CV) supplemented with 20% Fetal Bovine Serum (ATCC 30-2020). Adipocyte induction was performed using the Millipore Mesenchymal Adipogenesis Kit (Millipore SCR020), according to the manufacturer’s instructions. To assess adipogenesis the cells were stained with Oil Red O staining, followed by stain extraction, and quantification using a plate reader set to read absorbance at 520 nm.
Results
Foci were evident in the 3T3-L1 flaske after 3 weeks in culture at confluence demonstrating the loss of contact inhibition (Figure 1A, arrow). In contrast, the OP9 cells did not form foci after 3 weeks in culture at confluence indicating that contact inhibition was maintained (not shown).
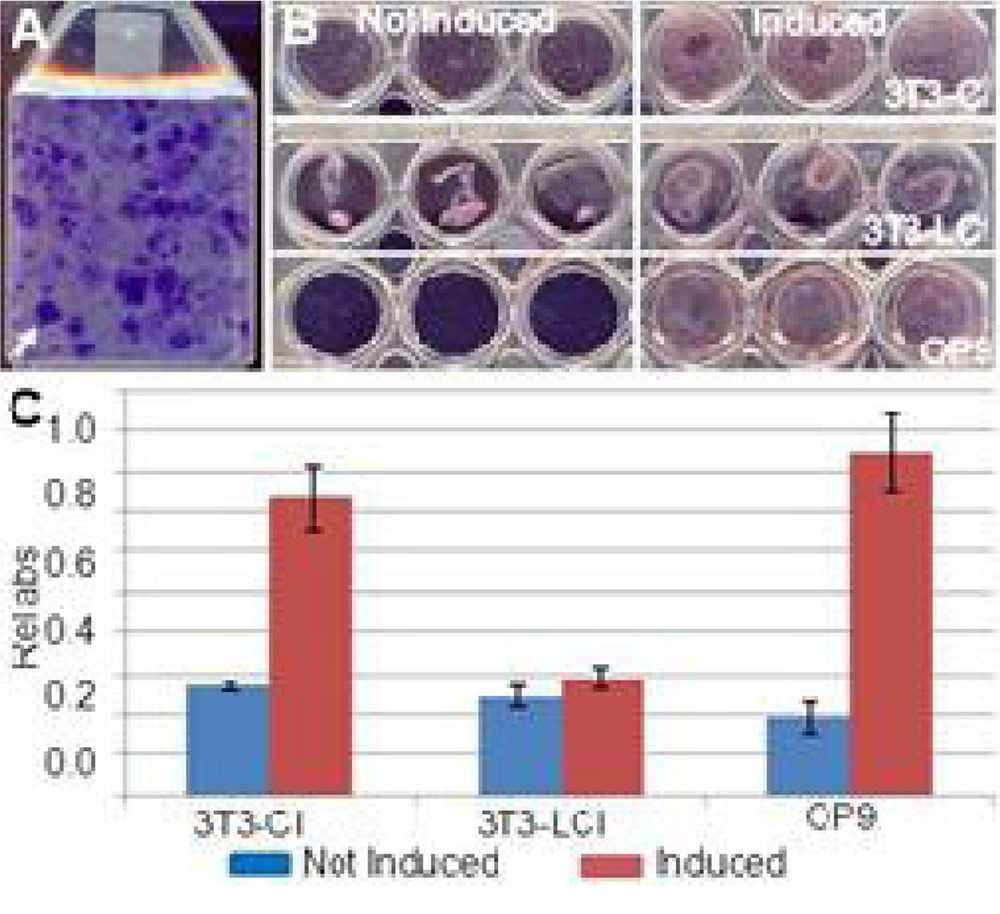
Figure 1. 3T3-LI cells lose contact inhibition and adipogenic potential. (A) Foci formation in 3T3-LI cells after 3 weeks at confluence. (B) Oil O Red staining of non-induced (left) and induced (right) 3T3-CI cells (top), 3T3-LCI (middle) and OP9 (bottom). (C) Quantification of (B).
To test if 3T3-L1 adipogenesis was dependent on contact inhibition, we compared the differentiation capacity of newly thawed, contact inhibited 3T3-L1 cells (3T3-CI) with cells that had been in culture at confluence for 3 weeks and had lost contact inhibition (3T3-LCI), and OP9 cells that had been cultured at confluence for 3 weeks concurrent with the 3T3-L1 cells. Based on Oil Red O staining, the 3T3-CI cells could be efficiently differentiated into adipocytes, while 3T3-LCI could not (Figure 1B, 1C)suggesting that 3T3-L1 adipogenesis is dependent on contact inhibition. Importantly, the OP9 cells differentiated into adipocytes as effectively as 3T3-CI cells (Figure 1B, 1C) indicating that the OP9 cell line is an acceptable substitute for the 3T3-LI cell line for applications requiring adipocytes.
Conclusions
We find that newly thawed, contact inhibited 3T3-L1 cells are adipogenic, but they lose adipogenic potential after being maintained in culture at confluence and losing contact inhibition. Thus, the differentiation potential of 3T3-L1 is dependent on contact inhibition. In contrast, we find the OP9 cell line does not lose contact inhibition after several weeks in culture at confluence and can be effectively differentiated into adipocytes. Therefore, the OP9 cell line is a suitable alternative to 3T3-L1 for studies requiring adipocytes.
Download a PDF of this application note
Download NowReferences
- T. H. Chang, and S. E. Polakis, ‘Differentiation of 3t3-L1 Fibroblasts to Adipocytes. Effect of Insulin and Indomethacin on the Levels of Insulin Receptors’, J Biol Chem, 253 (1978), 4693-6.
- N. E. Wolins, B. K. Quaynor, J. R. Skinner, A. Tzekov, C. Park, K. Choi, and P. E. Bickel, ‘Op9 Mouse Stromal Cells Rapidly Differentiate into Adipocytes: Characterization of a Useful New Model of Adipogenesis’, J Lipid Res, 47 (2006), 450-60.