Why TNBC is difficult to treat—and why PDOs matter
Unlike other cancer subtypes, TNBC lacks hormone receptors and HER2 amplification, rendering endocrine and HER2-targeted therapies ineffective. Standard treatment relies on cytotoxic chemotherapy, which often leads to resistance and relapse. The disease’s complexity is amplified by its molecular heterogeneity, with at least six distinct subtypes identified.
In a landmark study by Bhatia et al.,1 researchers created a biobank of long-term TNBC PDOs that recapitulate aggressive basal-like signatures. These PDOs were enriched in luminal progenitor-like cells (LP-like cells) with distinct transcriptional profiles and hyperactivation of NOTCH and MYC signaling—key drivers of tumor proliferation and survival.
Single-cell RNA sequencing revealed that these LP-like cells differ significantly from normal counterparts and exhibit tumor-intrinsic characteristics. Functional assays demonstrated that inhibition of these pathways using DAPT (NOTCH inhibitor) and MYCi975 (MYC inhibitor) significantly reduced organoid formation, suggesting therapeutic vulnerabilities.2,3
These findings confirm that the TNBC PDOs are powerful, physiologically relevant models that mirror the complexity of patient tumors and reveal new therapeutic targets—making them indispensable tools for advancing precision oncology.
The significance of patient-derived organoids (PDOs)
PDOs are three-dimensional cultures developed from stem cells isolated from patient tissues. These organoids preserve the genetic, phenotypic, and architectural features of the original tumor, making them powerful tools for cancer research, drug screening, and personalized medicine.
Unlike traditional 2-D cell lines, PDOs self-organize into complex structures and maintain cellular heterogeneity, offering a more accurate representation of tumor biology. They enable researchers to study tumor progression, mutational signatures, and treatment responses in a physiologically relevant context. PDOs can be derived from various tissue types and expanded long-term, allowing for the creation of biobanks that reflect diverse cancer subtypes and patient backgrounds.
One of the most compelling advantages of PDOs is their predictive power in drug response. Studies have shown that PDOs can forecast patient outcomes with high accuracy, making them ideal for tailoring therapies. Moreover, PDOs can be generated from both tumor and healthy tissues from the same patient, enabling the identification of drugs that selectively target cancer cells while minimizing toxicity.
PDOs also serve as platforms for genetic engineering using CRISPR-Cas9, allowing researchers to model early tumorigenesis and dissect the roles of specific mutations. When combined with decellularized extracellular matrix (dECM) scaffolds or organ-on-a-chip technologies, PDOs can replicate the tumor microenvironment, including stromal and immune interactions, further enhancing their translational relevance.
Despite challenges such as standardization and capturing full tumor heterogeneity, PDOs represent a significant advancement in cancer modeling. Their integration into preclinical workflows promises to accelerate drug development and improve patient-specific treatment strategies.
Breast cancer PDOs at ATCC
Through our collaboration with the Human Cancer Models initiative (HCMI), ATCC provides rare patient-derived 3-D organoid models that recapitulate the cellular morphology and heterogeneity of breast cancer tumors. These models offer researchers a transformative platform for studying tumor biology, drug response, and resistance mechanisms in a more clinically relevant context than traditional 2-D cell lines.
ATCC currently offers several expanded breast cancer models featuring distinct histopathology markers associated with HER2+, ER+, and/or PR+ subtypes. As TNBC PDO biobanks continue to grow and integrate genomic and immunological data, these models will become indispensable tools in clinical decision-making and translational research.
By faithfully modeling tumor biology, PDOs support precision drug screening, enable mechanistic studies of tumorigenesis, and facilitate the development of personalized therapies—representing a paradigm shift in how TNBC is studied and treated.
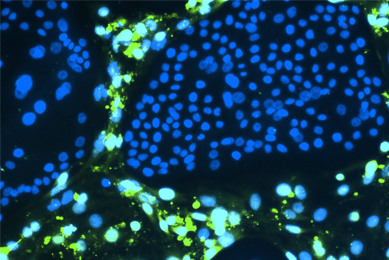
Breast cancer organoids (ATCC® PDM-411™) stained to visualize F-actin (green) and nuclei (blue)
Table 1: Breast cancer organoid models at ATCC
| Model Name | ATCC® No. | Tissue Status | Histology Subtype | HER2 | PR | ER | Stage |
|---|---|---|---|---|---|---|---|
| HCM-CSHL-0151-C50 | PDM-520™ | Primary | Infiltrating ductal carcinoma | — | + | + | IA |
| HCM-CSHL-0155-C50 | PDM-92™ | Primary | Other | + | — | — | IA |
| HCM-CSHL-0250-C50 | PDM-250™ | Primary | Pleomorphic carcinoma | — | + | + | IA |
| HCM-CSHL-0261-C50 | PDM-350™ | Primary | Infiltrating ductal carcinoma | — | + | IIA | |
| HCM-CSHL-0366-C50 | PDM-195™ | Primary | Metaplastic ductal carcinoma | — | — | + | IIB |
| HCM-CSHL-0655-C50 | PDM-411™ | Metastasis | Infiltrating ductal carcinoma | — | — | — | IIIA |
| HCM-CSHL-0773-C50 | PDM-523™ | Metastasis | Infiltrating ductal carcinoma | — | + | + | IA |
Table 2: Upcoming breast cancer organoid models
| Model Name | ATCC® No. | Tissue Status | Histology Subtype | HER2 | PR | ER | Stage |
|---|---|---|---|---|---|---|---|
| HCM-BROD-0965-C50 | PDM-648™ | Metastasis | Carcinoma | + | + | ||
| HCM-CSHL-0319-C50 | PDM-555™ | Primary | Infiltrating ductal carcinoma | + | |||
| HCM-CSHL-0440-C50 | PDM-556™ | Primary | Infiltrating ductal carcinoma | + | + | IA | |
| HCM-CSHL-0514-C50 | PDM-557™ | Primary | Lobular carcinoma | — | + | IIA | |
| HCM-CSHL-0907-C50 | PDM-605™ | Metastasis | Infiltrating ductal carcinoma | — | — | — | IIB |
Did you know?
ATCC offers over 230 organoid models spanning more than 20 tissue types.
Meet the authors
Abhay U. Andar, PhD
Lead Scientist, Microphysiological Systems, ATCC
Dr. Abhay U. Andar has over 13 years of experience in translational oncology, microfluidics, and advanced in vitro disease modeling. At ATCC, Dr. Andar leads the Human Cancer Model Initiative (HCMI) portfolio, which includes over 300 patient-derived cancer models spanning 28 indications. His research focuses on developing organoid systems to support therapeutic discovery and translational research in oncology. Dr. Andar earned his Ph.D. and M.Sc. in Biomedical Science and Engineering from the University of Glasgow, and a B.Sc. in Life Sciences from the University of Mumbai. He has authored numerous publications and patents in cancer research, microfluidics, and therapeutic manufacturing, with work featured in Nature Materials, Nature Biomedical Engineering, Lab on Chip, Cancer Research, and Biotechnology and Bioengineering. Dr. Andar’s innovations in Tumor-on-Chip platforms, organoid generation, and immune co-culture systems continue to shape the future of personalized medicine and drug discovery.
Stephen Friend, MS
Biologist, ATCC
Stephen Friend is a Biologist in the Microphysiological Systems group of the R&D department at ATCC. He started his scientific career at ATCC three years ago after receiving his Master’s Degree in Biomedical Science from Hood College. Stephen has worked on a variety of projects at ATCC including primary cell and cancer cell line culture, airway modeling and optimization using air-liquid-interface techniques, and the development and production of 3D patient-derived cancer organoids from various primary tissues.
Carolina Lucchesi, PhD
Principal Scientist, BioNexus, ATCC
Carolina Lucchesi is BioNexus Foundation Principal Scientist leading the Microphysiological Systems program at ATCC. Dr. Lucchesi received her PhD in Cellular and Molecular Biology from the University of Campinas in Brazil and has over 20 years of experience in Tissue Engineering and Organ-on-Chip technology. In her current role, Dr. Lucchesi leads the MPS program bringing new capabilities in the use of advanced 3D models and developing existing and new content to be applied in state-of-art technologies.
Explore our featured resources
Human Cancer Models Initiative (HCMI)
ATCC is the exclusive distributor of the Human Cancer Models Initiative (HCMI) models. See the models that include common and rare examples of cancer from numerous tissues.
More
Organoid Growth Kits
ATCC CoreKits are packages of recombinant proteins, small molecules and other supplements designed to make the preparation of complex media formulations for select cell culture models easy and reliable.
More Culture guide
Culture guide
Organoid Culture Guide
Get expert guidance on growing and maintaining organoids from our organoid culture guide.
MoreReferences
- Bhatia S, et al. Patient-Derived Triple-Negative Breast Cancer Organoids Provide Robust Model Systems. Cancer Res 82(7): 1174–1192, 2022. PubMed: 35180770
- Omar M, et al. Notch-Based Gene Signature for Predicting Chemotherapy Response in TNBC. J Transl Med 21: 811, 2023. PubMed: 37964363
- Priya P, et al. MYC Dysregulation in TNBC: Genomic Advances and Therapeutic Implications. 3 Biotech 15(1): 33, 2025. PubMed: 39777154