The theme for this year was “Advancing the Frontiers of Cancer Science and Medicine,” which brought together thousands of attendees spanning basic and clinical scientists, clinicians, patient advocates, and policy makers. The meeting encompassed educational sessions, method workshops, plenary sessions, poster sessions, and mini symposiums covering all aspects of basic and clinical cancer research with data updates on the ongoing clinical trials for the newest cancer therapies.
A big scientific focus in this year’s meeting was immuno-oncology and the growing need for advanced patient-derived models, which was evident by the breath of scientific sessions dedicated to showcasing the latest research in these areas. The “Embracing Immune Ecosystems” plenary session had some very exciting new data presented on myeloid cell immune checkpoints, the next generation of CAR-T cells, and immune cell heterogeneity and its impact in design and development of immunotherapy.
ATCC at AACR
The AACR exposition featured a wide array of companies showcasing their latest product offerings and capabilities for streamlining cancer discovery and research. The ATCC exhibitor booth showcased the newest and most cutting-edge offerings from ATCC, which included CAR-T target reporter cells, checkpoint reporter tumor cells, and new patient-derived models from the Human Cancer Models Initiative (HCMI) collection.
Chocolate Kingdom Reception
ATCC combined chocolate and science during our reception at The Chocolate Kingdom. With its interactive factory tour, chocolate museum, and river of chocolate, the Chocolate Kingdom provided a unique setting for a scientific presentation around innovative, advanced biological models. In the presentation, Jesse S. Boehm, PhD, CSO at Break Through Cancer, discussed how these models can be used to enhance the clinical relevance of oncology research and immunotherapeutic development. He focused on the importance of reducing the barriers between cancer research institutions and promoting data sharing among these institutions as strategies for speeding the pace of cancer drug discovery.
ATCC Exhibitor Spotlight Session
This workshop highlighted two application areas of ATCC’s current focus: 2-D and 3-D models from the HCMI collection and reporter models and primary cells for immuno-oncology–based therapeutics.
Utsav Sharma, PhD, Product Manager at ATCC, kicked off the presentation by introducing our overarching focus on oncology product areas. Dr. Sharma related how various fields of cancer drug discovery and development—such as biomarker discovery, tumor biology, and drug screening—are supported by the breadth of ATCC’s product offerings.
Next, Hyeyoun Chang, PhD, Scientist at ATCC, addressed ATCC’s immuno-oncology program. After introducing this new corporate initiative, Dr. Chang discussed some of the new products that we are generating to support this growing area of cancer therapy. Some of the product areas that she focused on included human primary immune cells, CAR-T target luciferase reporter cells, checkpoint luciferase reporter cell lines, and THP-1 reporter cells.
Last, James Clinton, PhD, Lead Scientist at ATCC, presented ATCC’s involvement in the HCMI, an international consortium that is dedicated to generating novel human tumor-derived culture models with associated genomic and clinical data. Dr. Clinton described the features of advanced 2-D and 3-D models such as organoids, neurospheres, and conditionally reprogrammed cells. He highlighted the importance of these models by describing the breadth of sequencing and patient clinical data accompanying these models and how these data can be accessed for free by the research community.
ATCC Poster Session
In addition to the Exhibitor Spotlight Session, Dr. Chang presented a scientific poster around ATCC’s checkpoint luciferase reporter cell lines titled “Immune Checkpoint Reporter Cell Lines Based on the Protein Profiling of ATCC Cell Lines for Cancer Immunotherapy Drug Screening.” In her presentation, Dr. Chang showed how these novel cell lines can be incorporated into simple blocking assays or integrated into sophisticated co-culture cell-based drug screening assays. You can watch the presentation on demand or download the poster if you missed the poster session and are interested in seeing the compelling data.
ATCC-NCI HCMI Joint Session
Dr. James Clinton along with members from NCI’s Center for Cancer Genomics (CCG) conducted a joint session on the HCMI project titled “Human Cancer Models Initiative: A Resource of Next-generation Cancer Models and Data.” At this session, the presenters provided an overview of the HCMI project, demonstrated how to navigate the HCMI searchable catalog and associated patient-derived model data, and highlighted ATCC’s role as the exclusive distributor of these models.
What ATCC can offer
Cancer patients rely on the efforts of the pharmaceutical industry, biotechnology firms, and academic institutions to develop the therapies needed to treat both common and rare forms of the disease. Yet, the lack of available models relevant to tumor type, disease progression, and population diversity impedes our progress toward rapid advancements in translational medicine.
ATCC can help by providing authenticated cancer models that lead to breakthroughs in translational research. To this end, we produce the materials and standards that scientists need for drug screening, tumor mechanism studies, biomarker discovery, cancer immunology, and cancer diagnostics development. New models such as organoids, conditionally reprogrammed cells, neurospheres, and luciferase-expressing reporter cell lines are essential for your cutting-edge cancer research.
Did you know?
ATCC provides resources such as application notes, scientific posters, and webinars that focus on cancer diagnostic and drug discovery and development applications.
Utsav Sharma, PhD
Product Manager, Oncology, ATCC
Dr. Utsav Sharma is the Product Manager for the Oncology portfolio at ATCC. Utsav obtained his PhD in Cancer Biology at the University of Miami School of Medicine, and completed his postdoctoral training at Georgetown University, focused on the tumor microenvironment, metastasis, liquid biopsy, and clinical cancer research. Prior to ATCC, Utsav worked as a Senior Scientist at Autolus Therapeutics PLC on their CD19 CAR-T platform Obe-cel, providing technical and scientific oversight to the global phase-II clinical trial on adults with Acute Lymphoblastic Leukemia (ALL) disease. Utsav has a passion for cell-based research and aims to use his scientific and business development background to partner with clients to accelerate their work using the next generation services and product offerings from ATCC.
Brian Shapiro, PhD
Marketing Segment Manager, Oncology, ATCC
Brian A Shapiro, PhD, works to communicate the scientific breakthroughs of ATCC’s product development laboratories to the biomedical research community. Brian is the Executive Producer of ATCC's Podcast, Behind the Biology. Previously, he worked at Virginia Commonwealth University, where he investigated the role of pre-mRNA splicing in the multi-drug resistance of lung cancer. Dr. Shapiro attended the Medical College of Georgia, where his research focused on adrenal physiology as well as diseases of the epidermis.
Explore our resources
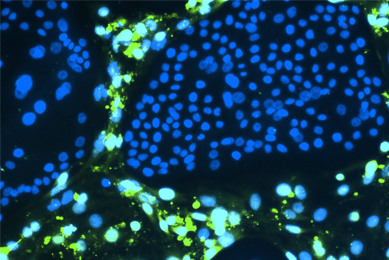
Human Cancer Models Initiative (HCMI)
ATCC is the exclusive distributor of the Human Cancer Models Initiative (HCMI) models. See the models that include common and rare examples of cancer from numerous tissues.
More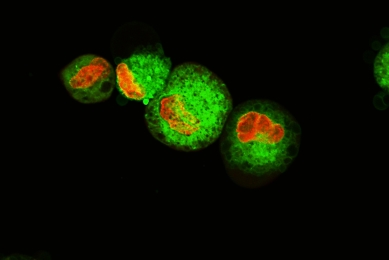
CAR-T Target Luciferase Reporter Cells
ATCC generated luciferase reporter solid and liquid tumor cell lines that express tumor antigens such as CD19, CD20, and HER2. Learn how you can incorporate these target cells into your T-Cell cytotoxicity assays to enable your breakthroughs in immuno-oncology.
More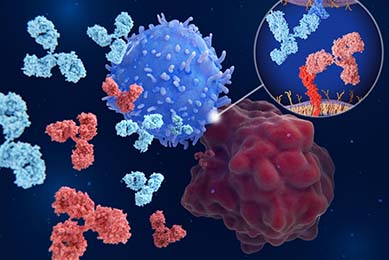
Checkpoint Luciferase Reporter Cells
ATCC created tumor and immune cell lines with high endogenous expression of checkpoint inhibitory and co-stimulatory expression levels. Learn how you can integrate these reporter cells in the development of new checkpoint inhibitors for immuno-oncology drug discovery.
More