
Authors: Fang Tian, PhD, and John Foulke, MS
Animal models are essential tools for evaluating the therapeutic effects of anticancer drug candidates in preclinical studies and investigating the mechanism of tumor development in basic cancer research. However, the current techniques used to assess tumor growth in these models are unwieldy due to animal maintenance and tumor measurement limitations. Therefore, alternative approaches that are both non-invasive and accurate are needed. Here, we will review the current models and techniques used in cancer research and we will discuss the use of bioluminescence imaging for the precise histological measurement of tumors.
Currently, mouse xenograft models are the most frequently used animal models for assessing the antitumor effect of oncology drugs due to the ease of initiating tumor growth and measuring tumor burden. Establishing a xenograft model typically involves the subcutaneous inoculation of human cancer cells into immunocompromised animals.1 A researcher can monitor tumor growth by measuring tumor length and width with a caliper; these measurements are then used to calculate tumor volume. While this method provides a visual confirmation of tumor development before initiating therapy, the resulting data may be subject to error as caliper-based measurements can be affected by observer subjectivity and variations in tumor shape and compressibility.2
Orthotopic animal models are also commonly used in cancer research as they recapitulate many of the essential, clinically relevant features of tumor growth. Here, cancer cells are inoculated into the equivalent organ from which the cancer originated.1 After inoculation, tumor growth is traditionally assessed by sacrificing groups of experimental animals at different time points and comparing tumor size. Because of the large number of animals and laborious efforts required, the use of orthotopic models is less efficient and more costly than subcutaneous xenograft models.
In recent years, several noninvasive imaging methods for measuring tumor burden in small animals have become available. These methods provide an attractive, cost-effective approach for animal studies as they considerably reduce the number of experimental animals and enable the continuous evaluation of tumor growth over the course of multiple stages of tumor development.3 Of the current noninvasive methods available, bioluminescence imaging (BLI) has rapidly gained popularity in cancer research as it provides reliable and sensitive real-time imaging of small tumor lesions without the need for an external excitation light source.4-6
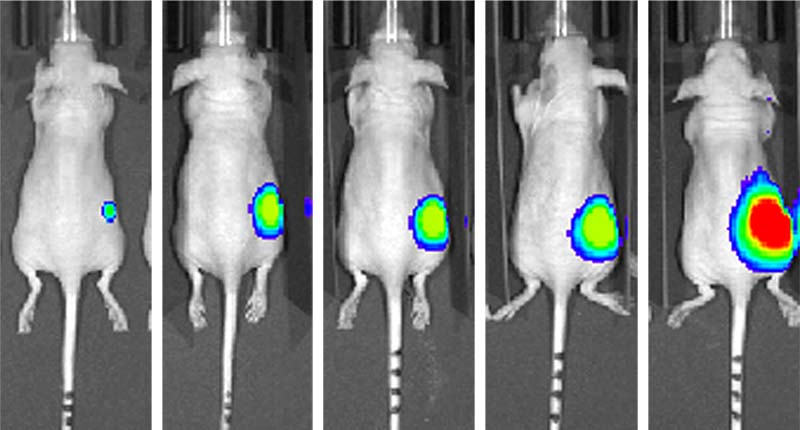
Bioluminescence imaging techniques enable reliable and sensitive real-time imaging of small tumor lesions.
The mechanism of BLI is based on light-generating enzymes such as firefly luciferase. In this ATP-dependent reaction, luciferase catalyzes the substrate luciferin into the light-emitting product oxyluciferin.4,5 While many invertebrates, bacteria, and protists express luciferase, isoforms of this enzyme are not harbored in mammalian genomes; therefore, the first step in generating BLI models is to establish reporter-labeled cancer cell lines that stably express the luciferase protein. The luciferase-reporter cancer cells are then inoculated into experimental animals and bioluminescence measurements are assessed via in vivo imaging equipment after the administration of luciferin. Although the use of BLI in cancer research has increased substantially in recent years, there is a limited number of credible, commercially available luciferase-reporter cancer cell lines that can be used to create BLI animal models.
To support this need, ATCC has created a portfolio of well-authenticated reporter cell lines that express high levels of firefly luciferase. Here, a set of commonly used human or mouse tumor cell lines representing different tissue types were selected for genetic engineering on the basis of their scientific relevance and their capacity for establishing tumors in animals. As luciferase expression level and stability are the key considerations for a successful BLI model, ATCC used lentivirus transfection and single cell cloning approaches to isolate stable clones with high luciferase expression. The luciferase-expressing cell lines were authenticated at the phenotypic, genomic, and functional levels; here, cell lines were extensively characterized via cell morphology examination, growth kinetics studies, STR profiling, mouse pathogen testing, bioluminescence signal quantification, and in vivo live animal BLI analysis. Overall, these luciferase-expressing reporter cell lines provide cancer researchers with a complete set of reliable, easy-to-use tools that support whole-animal bioluminescent imaging studies.
Animal models have a long history as essential components in cancer research; however, the application of these models is often limited by the techniques used for evaluating tumor growth and burden. In recent years, the use of BLI in translational research has substantially increased as this technology enables the visualization of in vivo biological events, reduces the number of experimental animals, allows for continual monitoring/imaging of a single individual animal, and decreases the amount of interanimal variation. To support the use of this technology, ATCC has created an expanding portfolio of reporter cancer cell lines that express high levels of firefly luciferase. These advanced models provide a relatively simple, robust, and highly sensitive means to measure biological processes and assess drug efficacy in animal models through bioluminescence imaging, thereby successfully bridging the gap between in vitro and in vivo research.
Download a PDF of this white paper
Download NowReferences
- Jung J. Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicol Res 30(1): 1-5, 2014.
- Jensen MM, et al. Tumor volume in subcutaneous mouse xenografts measured by microCT is more accurate and reproducible than determined by 18F-FDG-microPET or external caliper. BMC Med Imaging 8: 16, 2008.
- Koo V, Hamilton PW, Williamson K. Non-invasive in vivo imaging in small animal research. Cell Oncol 28(4): 129-139, 2006.
- Close DM, et al. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel) 11(1): 180-206, 2011.
- Zinn KR, et al. Noninvasive bioluminescence imaging in small animals. ILAR J 49(1): 103-115, 2008.
- Choy G, et al. Comparison of noninvasive fluorescent and bioluminescent small animal optical imaging. Biotechniques 35(5): 1028-1030, 2003.