Genetically Modified Human Renal Proximal Tubule Epithelial Cells (RPTEC/TERT1): A New Model for Drug Toxicity Studies Webinar
February 23, 2017, at 12:00 PM ETAbstract
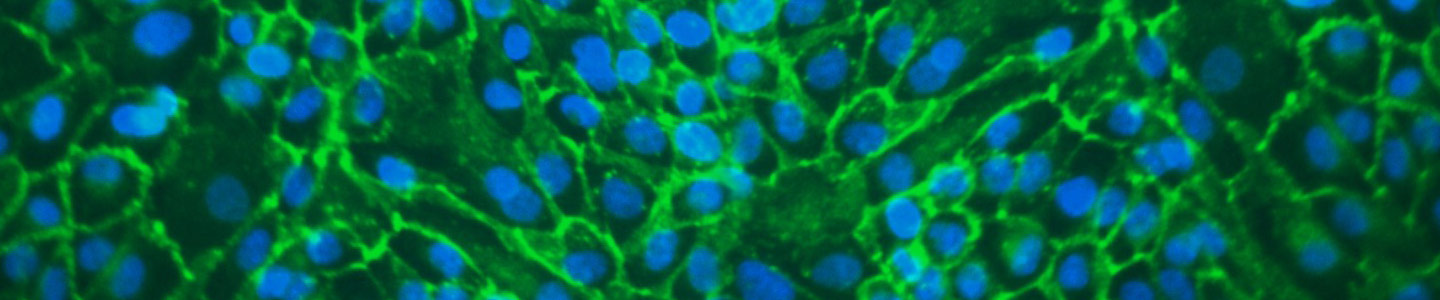
In vivo studies have shown that kidney membrane transporters play a key part in drug disposition and renal clearance. Primary renal proximal tubule epithelial cells (RPTEC) are the most physiologically relevant cell models, but lose OAT1, OCT2, and OAT3 transporter expression in culture. In addition, primary RPTEC transiently expressing these transporters show large variations between production lots, making the data hard to interpret. Furthermore, cell line-based models either do not have the kidney tissue origination or the cell line itself is a cancer line.
This presentation will introduce transporter cell models using a well characterized hTERT-immortalized RPTEC that stably overexpress either the OAT1, OCT2, and OAT3 gene. Our data show that these modified cell lines are very useful tools that provide kidney tissue-relevant results, improved consistency over time, and more predictability for clinical trials versus current models.
Key Points
- Kidney membrane transporters play a key part in drug disposition and renal clearance, however there is a lack of in vitro models that durably and correctly recapitulate kidney physiology
- ATCC has created kidney cell models using a well characterized hTERT-immortalized RPTEC that stably overexpress the OAT1, OCT2, or OAT3 gene
- Our data shows that these modified cell lines are very useful tools that provide kidney tissue-relevant results, improved consistency over time, and predictability for clinical trials
Presenter
Chaozhong Zou, PhD
Senior Scientist, ATCC Cell Systems
Dr. Chaozhong Zou is a Senior Scientist and the Group Leader of the Immortalized Cells group at ATCC Cell Systems. Dr. Zou has extensive experience in cell-related product development such as immortalized cell lines, stable reporter cell lines, and cell-based assays development. Dr. Zou was a research scientist at NorthShore University HealthSystem, University of Chicago Medical School and conducted postdoctoral research at Northwestern University and Tufts University.
Questions and Answers
Can these cells be cultured in a polarized way with a clear separation of apical vs. basolateral markers?
The parental line RPTEC/TERT1 displays clear separation between apical and basolateral markers. We are planning to perform the same experiments in the RPTEC/TERT1-OAT1 and RPTEC/TERT1-OCT2 cell lines.
Do these cells express plasma membrane receptors such as megalin, cubilin, and amnionless?
We have not stained for these receptors yet.
Do you have transepithelial electrical resistance (TEER) data for these two cell lines? Have you tried expressing both OAT1 and OCT2 in the same cell line?
We have not performed the TEER experiments yet. In addition, we have not co-expressed OAT1 and OCT2 in the same cell line.
Do you need a special surface to grow the RPTEC/TERT1-OAT1 and RPTEC/TERT1-OCT2 cell lines? What media do you recommend using to culture them?
These cells will attach and grow on cell culture dishes, flasks, or plates treated for adherent cells. We recommend culturing the cells in DMEM: F12 (ATCC 30-2006) supplemented with the hTERT RPTEC Growth Kit (ATCC ACS-4007).
Do you need special media to culture the RPTEC/TERT1-OAT1 and RPTEC/TERT1-OCT2 cell lines?
You can use the same medium as the parental RPTEC/TERT1 cell line (ATCC CRL-4031), simply add hTERT-immortalized RPTEC Growth Kit (ATCC ACS-4007) to Dulbecco’s Modified Eagle’s Medium (DMEM: F12; ATCC 30-2006). If you want to culture the cells continuously, we suggest that you add 0.3ug/mL puromycin for selection pressure.
Does ATCC have the control RPTEC/TERT1 cell line (ATCC-CRL-4031) as well?
Yes, the cell line is available on the ATCC website under the catalog number ATCC CRL-4031.
Have you looked at other transporter such as OCTN1, OCTN2, OATP4C1?
When we investigated OATP4C1 expression in the RPTEC/TERT1 cells, we found that the gene is expressed at a lower level than that of primary cells. We have not yet analyzed cells for OCTN1 and OCTN2 expression.
Have you used any other substrate to validate your RPTEC SLC transport cells?
We are planning to perform that analysis. We used fluorescence-based assays as the starting point because the assay itself is simple to perform, did not require sophisticated instruments, and is technically less challenging.
How is this model different from the OAT1 HEK 293T/17 (ATCC CRL-11268G-1) cell model?
The major difference is that OAT1 HEK 293T/17 was derived from an embryonic kidney cell line (HEK 293T/17; ATCC CRL-11268) that was transformed by an adenovirus. In contrast, the RPTEC/TERT1 (ATCC CRL-4031) cell line that was featured in the webinar was derived from adult kidney proximal tubule cells. In mammalian physiology, the renal tubule is where the actual organic anion and cation uptake occurs. Therefore, the RPTEC/TERT1 transporter models will be more relevant to the in vivo situation and will have more predictability for clinical testing than embryonic cell-based models.
How long can the RPTEC/TERT1-OAT1 and RPTEC/TERT1-OCT2 be passaged?
RPTEC/TERT1-OAT1 and RPTEC/TERT1-OCT2 were confirmed at ATCC to be functional at passage 10.
Is the level of OAT1 comparable to endogenous OAT1 in primary proximal tubule cells? Does the overexpression of OAT1 affect the response to drugs and molecules?
We have not performed this comparison yet. However, our available drug testing data has indicated that the overexpression of OAT1 does not affect the drug response.