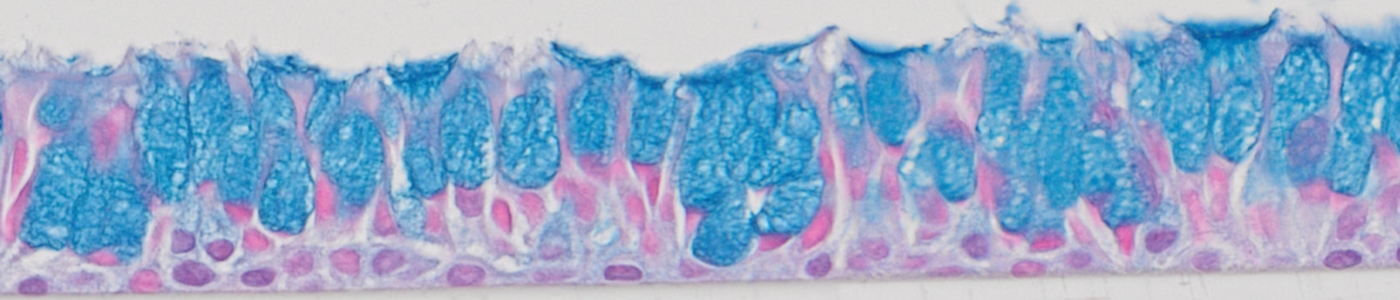
Evaluating airway ALI model fabrication methods and comparing differentiation potential of primary and hTERT-immortalized epithelial cells
Cell Bio 2022
Washington, DC, United States
December 04, 2022Abstract
Download the poster to learn about this optimal method of fabricating airway models
DownloadWatch the poster presentation
Presenter
Kevin Tyo, PhD
Scientist, ATCC
Dr. Kevin Tyo is a Scientist in Research and Development at ATCC with over 10 years of experience in biological research. In his current role, Dr. Tyo develops and evaluates advanced in vitro co-culture models, as well as conducts toxicological testing. Dr. Tyo received his Ph.D. in Pharmacology and Toxicology from the University of Louisville in 2019, where he designed and tested topical drug delivery platforms that provided sustained release of antiviral therapeutics.
Toxicology testing products
ATCC knows that worthwhile science takes time, especially in the toxicology, pre-clinical stages of drug development. It is critical that the standards and model organisms used in toxicological testing are reliable and authenticated. We can help streamline your research by providing the most authenticated, advanced, and functional models available. Let ATCC revolutionize and accelerate your toxicology studies in every phase of the research and testing process.
ATCC provides the cells, media, and reagents needed to explore each step of the in vitro preclinical testing process—from modeling, screening, and characterization to exploratory toxicology to pharmacokinetics and metabolism. We provide renal, neural, airway, and skin models for such applications as high-content screening, 3D culture, spheroid culture, permeability assays, metabolic stability and survival studies, transport activity measurement, and more.
Explore toxicology tools