Mycoplasma contamination affects roughly 15-35% of continuous cell cultures.1,2 The deleterious effects of these types of contamination include the induction of chromosomal abnormalities, the disruption of DNA and RNA synthesis, and the inhibition of both cell metabolism and growth rate. Therefore, mycoplasma contamination constitutes a serious concern for cell culturists.1,3,4
Download a PDF of this application note
Download NowMycoplasmas are classified as Mollicutes, which are a distinct class of bacteria distinguishable by their small size (ranging from 0.2 to 0.8 μm) and complete lack of a cell wall. Over 190 species of mycoplasma have been identified, but of these, just 8 are responsible for approximately 95% of all cell culture contaminating events.4,5 Commonly, contamination occurs through a cross contaminating event, such as occurs when laboratory personnel handle both clean and contaminated media or cell cultures at the same time.1 Importantly, these contaminating events may be difficult to detect or prevent. For example, mycoplasma can grow to densities of 107-108 organisms/mL without affecting obvious changes in the turbidity or pH of the culture media.6 Further, they have a small genome (580 kb to 2,220 kb) and correspondingly few metabolic pathways that are active enough to reveal their presence.6 Furthermore, because mycoplasma do not possess a cell wall, the majority of commonly used antibiotics are not effective at preventing their growth should a contaminating event occur. Therefore, the best protection against mycoplasma is to identify a contaminated culture quickly, before the contamination spreads to other cultures. To facilitate the rapid and reliable detection of mycoplasma in cell culture, ATCC offers the PCR-based Universal Mycoplasma Detection Kit (ATCC 30-1012K).
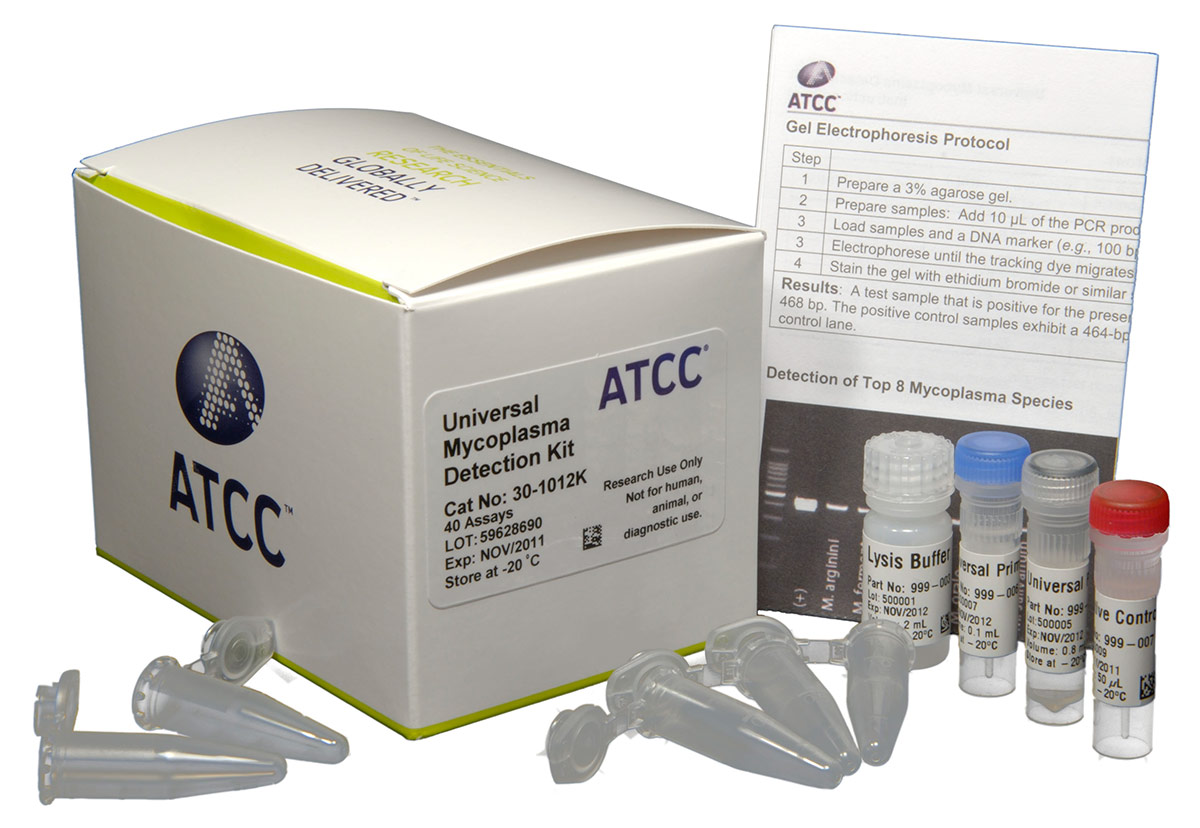
Universal Mycoplasma Detection Kit (ATCC 30-1012K)
The Universal Mycoplasma Detection Kit is able to detect a wide range of Mollicutes infections, including the 8 species most likely to contaminate cell cultures (M. arginini, M. fermentans, M. hominis, M. hyorhinis, M. orale, M. pirum, M. salivarium, and A. laidlawii), as well as species from other Mollicutes genera like Acholeplasma, Spiroplasma, and Ureaplasma. To achieve detection over a wide range of species, the kit uses universal primers specific to the conserved 16S rRNA coding region of the mycoplasma genome, a thermo-stable Taq-polymerase, and a touchdown PCR approach.7 The touchdown PCR protocol employs a high annealing temperature in the initial cycle that decreases with subsequent cycles. This protocol increases the likelihood of primers binding to the specific targets and reducing the likelihood that nonspecific targets (ie, from cells or other bacteria like E. coli) will be amplified. Using this method, mycoplasma contamination is easily recognized as a distinct PCR product ranging in size from 434 to 468 bp on an agarose gel, and the assay is sensitive to as few as 20 genome copies.
The Universal Mycoplasma Detection Kit comes complete with a proprietary mix of primers, buffers, dNTPs and thermo-stable polymerase that have been optimized to generate the consistent, reliable results, in a convenient and easy to use format. The kit has everything you need to keep mycoplasma contamination from spreading through your cell cultures, and affecting your research.
Download a PDF of this application note
Download Now
References
- Drexler, H.G. & Uphoff, C.C. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology 39, 75-90 (2002).
- McGarrity G. J., S., T., and Vanaman, V. (ed.) Detection of Mycoplasmal Infection of Cell Cultures by DNA Fluorochrome Staining. (Academic Press, New York, 1983).
- McGarrity, G.J., Constantopoulos, G. & Barranger, J.A. Effect of mycoplasma infection on pyruvate dehydrogenase complex activity of normal and pyruvate dehydrogenase complex deficient fibroblasts. Exp Cell Res 151, 557-62 (1984).
- Rottern, S. Interaction of Mycoplasmas with Host Cells. Physiology Review 83, 15 (2003).
- Barile, M.F. Mycoplasmal flora of simians. J Infect Dis 127, Suppl:S17-20 (1973).
- Razin, S., Yogev, D. & Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev 62, 1094-156 (1998).
- Eldering, J.A., Felten, C., Veilleux, C.A. & Potts, B.J. Development of a PCR method for mycoplasma testing of Chinese hamster
ovary cell cultures used in the manufacture of recombinant therapeutic proteins. Biologicals 32, 183-93 (2004).